iMiGiNE, a paradigm shift in the production of radiopharmaceuticals for the medicine of tomorrow
iMiGiNE, a paradigm shift in the production of radiopharmaceuticals for the medicine of tomorrow
PMB and CEA Announce the Commissioning of iMiGiNE at Frederic Joliot Hospital (Orsay), Paving the Way for Next Generation Molecular Imaging Diagnostics Tweet
A change in paradigm and scale: in late 2020, the iMiGiNE system for “on-demand” automated microfluidic production of radiopharmaceuticals for medical imaging began operation. This world first—the culmination of nearly 10 years of collaboration between PMB and the CEA (Alternative Energies and Atomic Energy Commission) and supported by Bpifrance—took place at the Service Hospitalier Frédéric Joliot research center (CEA, Orsay Hospital), where the system is installed. This demonstration opened up new possibilities for providing radioactive molecules for personalized molecular imaging and related care.
Peynier, France, 12th May 2021 - On December 10, 2020, the iMiGiNE system was used for the fully automated production of the first two syringes of 18F-sodium fluoride (18F-NaF), a radiopharmaceutical used to detect bone metastases in the initial stage or during the monitoring of bone recurrences in certain cancers. Beyond this first proof of concept, the project’s research teams are laying the foundations for the nuclear molecular imaging of the future, as close as possible to patient needs.
Unlocking the technological door to personalized medicine
Many technological challenges arose in miniaturizing, automating, and simplifying the radiopharmaceutical production chain before achieving this result. Currently, only about ten of the radiopharmaceuticals used in PET imaging1 have marketing authorization in France, while about a hundred molecules that would facilitate more accurate diagnosis than the marketed molecules remain undeveloped. Why? The cumbersome infrastructure, the technical nature of the manufacturing operations, and the very short radioactive half-life of the radioactive isotopes used (110 minutes for fluorine-18 and only 20 minutes for carbon-11). This is one of the reasons why manufacturers have solely focused on a few fluorine-18 radiolabeled molecules. Carbon-11, which has an even shorter half-life, is used exclusively by a few expert centers.
By developing iMiGiNE, the first fully automated, compact system that brings together all the elements of the radiopharmaceutical production chain, the teams at the CEA's SHFJ research center2 and PMB are putting the molecule back at the heart of nuclear imaging diagnosis. The device’s ease of use and modularity allow it to be rolled out more broadly than a standard production chain and to move from “batch” manufacturing to rapid, “on-demand” manufacturing of the radiopharmaceutical that is best suited to each patient.
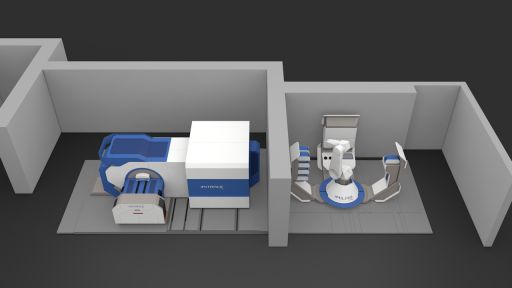
How does iMiGiNE work?
Installation and assembly of the various iMiGiNE modules began in 2019 and was completed in late 2020. The system includes a cyclotron to produce the radioisotopes, an automated radiochemistry machine operating on a microfluidic scale to produce the radiopharmaceutical, a syringe filling unit, and a quality control system, all managed by a robotic arm. iMiGiNE is compact and automated, making it fast and easy to produce radiopharmaceuticals for the patient from cyclotron to syringe.
1. The cyclotron
The iMiGiNE cyclotron is designed with a superconducting magnet, which gives it great stability and a limited footprint. It is lighter and more compact than a “conventional” cyclotron. The casemate, a room built around the cyclotron, also benefits from streamlined engineering.
2. The miniature radiochemistry laboratory
iMiGiNE is equipped with an iMiLAB® radiosynthesis controller that was designed and built to be used in R&D as well as in radiopharmaceutical production. The operating principle relies on dedicated cassettes containing all the ingredients needed to produce the chosen radiopharmaceutical. These cassettes are single use to limit the risk of cross-contamination from one batch to another, a fundamental principle in drug manufacturing. Operating on a microfluidic scale, this device optimizes yields while reducing production time, which is a key parameter given the half-life of the isotopes used. Using only volumes that do not exceed a few hundred microliters, this controller uses reagents and solvents sparingly. The control interface makes it possible to develop specific programs for each radiopharmaceutical, thus increasing the number and variety of products. This fast and versatile radiosynthesis device integrated with iMiGiNE brings the innovative concept of “dose on demand” to life, adapting to the needs of each patient.
3. A robotic arm to manage production
From positioning the microfluidic cassette in the radiosynthesis controller to placing the syringes in a dedicated area, disposing of production waste, and positioning a new microfluidic cassette, the entire manufacturing cycle is managed by a robotic arm that can repeat these operations several times a day and offer a new radiopharmaceutical drug with every cycle. This series of operations requires no human intervention, making it possible to carry out a series of productions while following the rules of radiation protection for users. The seamless execution of all these operations allowed us to prepare those two syringes of 18F-NaF. The next steps will involve expanding the range of available radiopharmaceuticals radiolabeled with fluorine-18 and carbon-11.
4. Automatic syringe filling
Based on a CEA patent, the iMiGiNE device automatically sterilizes the radiopharmaceutical preparation, which ensures microbiological quality, and then prepares two syringes, the first containing the dose needed for the patient and the second for quality control purposes.
Press contacts:
Fatine Slaoui – [email protected] - +33 4 42 53 13 13
https://www.imigine.com/contact
About the CEA
The CEA is a key player in research, supporting the State, the economy, and the general public. It provides concrete solutions to their needs in four main areas: energy transition, digital transition, technologies for the medicine of the future, and defense and security.
The CEA conducts its primary research activities in the fields of biotechnology and health, the science of matter and the Universe, physics, and nanosciences. Producing and publishing knowledge and expertise at the highest international level is central to its objectives. In 2019, nearly 3,800 scientific publications—three quarters of them resulting from international collaborations—were authored by CEA researchers. This knowledge is also an indispensable resource for the CEA’s other missions.
The Service Hospitalier Frédéric Joliot research center, affiliated with the CEA's Joliot Institute, was established in 1958 within the Orsay Hospital (south of Paris) to develop isotopic medical imaging. Today, it is one of the only research units in Europe that combines the various molecular and functional imaging methods dedicated to preclinical and clinical research, while also having both basic research laboratories and a clinical nuclear medicine unit.
A pioneer in the production of short-lived radioisotopes, the SHFJ is a leader in PET imaging developments. It develops innovative radiopharmaceuticals and supports their transition to clinical applications. For 10 years, from 1998 to 2008, the SHFJ was responsible for 100% of the Île-de-France production of 18FDG—the fluorine-18 labeled glucose that has revolutionized cancer diagnosis—thus giving industry time to invest in cyclotrons to take over. The service supported that transition, as well as the massive expansion of PET in hospitals.
In recent years, the CEA patented the synthesis of two original radiopharmaceuticals before transferring them to industry: 18F-LBT-999 for the diagnosis of Parkinson's syndrome and 18F-fludarabine for the diagnosis of hematologic malignancies.
About PMB
For over 20 years, PMB's core business has been designing and manufacturing high-tech assemblies and systems for medicine (components for X-ray tubes used in CT scans, components for radiotherapy machines, linear particle accelerators, etc.), research, nuclear energy, defense & security, and industry. PMB is part of the French industrial group ALCEN.
The company has been developing, designing, and manufacturing its own medical systems since 2012.
The first, iMiGiNE, is an automated production system for injectable radiopharmaceuticals for PET imaging. It combines:
- iMiTRACE, a 12 MeV cyclotron with a superconducting magnet.
- iMiLAB, a robotic radiochemistry room allowing the combination of a radioisotope with a molecule of clinical interest.
By providing solutions to current limitations in the way radiopharmaceuticals are produced, the iMiGiNE system enables medical centers to open up new possibilities in the fields of diagnosis and research.
The second device designed and manufactured by PMB, FLASHKNiFE®, is a FLASH radiotherapy system designed to irradiate cancer cells without side effects on neighboring healthy cells. In 2020, PMB announced the launch of this very promising system that is currently undergoing clinical trials.
Glossary (or insert)
Radioactive half-life.
Radioactive half-life is a physical quantity specific to each radioactive isotope which measures—in seconds, minutes, hours, days, or years, depending on the radioelement—the time after which half of the radioactive nuclei present at a given moment have decayed. The half-life of fluorine-18 is 109.8 minutes, while that of carbon-11 is 20.4 minutes. Among the radioactive isotopes used in medical imaging, oxygen-15 is one of those with the shortest half-life (2 min) while zirconium-89, on the other hand, has a half-life of 3.2 days.
Radiopharmaceutical drug.
A radiopharmaceutical drug is a drug that contains at least one radioelement among its constituent atoms. This radioisotope is introduced by radiosynthesis. Depending on the radioisotope’s physical decay properties, the radiopharmaceutical may be used for:
- Diagnosis with positron emission tomography, if it is radiolabeled with a positron emitter (for example, 18F-NaF radiolabeled with fluorine-18).
- Diagnosis with single-photon tomography if it is radiolabeled with a gamma emitter (for example, Neurolite radiolabeled with technetium-99m).
- Vectorized radiotherapy if it is radiolabeled with a β- or α emitter (for example, Luthatera radiolabeled with lutetium-177).
1 Positron Emission Tomography: a medical imaging technique used for diagnosis. Like scintigraphy and vectorized radiotherapy, it is a nuclear medicine examination; it uses radioactive molecules called radiopharmaceuticals.
2 Service Hospitalier Frédéric Joliot research center, affiliated with the Frédéric Joliot Institute of Life Sciences. Pages/research_entities/SHFJ.aspx