
| Media Relations: | Investor Relations: |
|---|---|
| Ashleigh Koss | Felix Lauscher |
| 908-981-8745 | +33 (0)1 53 77 45 45 |
| Email: [email protected] | Email: [email protected] |







Media Panel
Resources
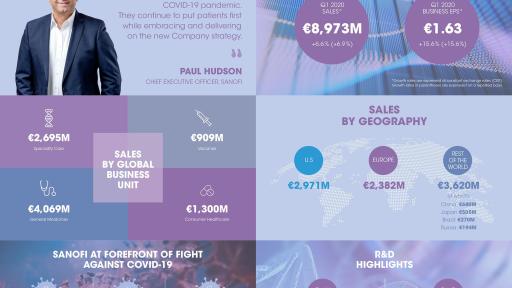
Sanofi at forefront of fight against COVID-19 in Q1 2020
Rapid and decisive response to COVID-19 global health crisis
- Sanofi showed resilience and maintained full business continuity worldwide including its global industrial network.
- Separate collaborations announced with BARDA, Translate Bio and GSK to develop novel COVID-19 vaccines.
- Global clinical program underway to evaluate Kevzara® in patients hospitalized with severe COVID-19.
- Initiated two studies evaluating hydroxychloroquine as a treatment for COVID-19. Commitment to donate 100 million doses.
- In addition, a leading European API company to be created to help balance the industry’s reliance on API sourced from Asia.
Q1 2020 sales growth performance driven by Dupixent®
- COVID-19 stocking in channels explains about half of company sales growth in Q1; Dupixent® growth unaffected by COVID-19.
- Net sales were €8,973 million, up 6.9% on a reported basis and 6.6%(1) at CER with Dupixent® sales up 129.8% to €776 million.
- Specialty Care sales up 31.3% driven by Dupixent® performance and double-digit growth of Aubagio® and Rare Disease.
- Vaccines sales increased 3.7% reflecting high base for comparison and decline in the travel category.
- General Medicines sales decline moderated (-3.8%) due to demand for chronic therapies including Diabetes (-1.2%).
- CHC sales up 4.2% driven by performance in Rest of World region and supported by additional demand related to COVID-19.
- China sales (-14.4%) impacted mainly by VBP program, partly offset by significant volume gains for Plavix® and CoAprovel®.
Q1 2020 business EPS(2) growth reflected underlying performance and COVID-19 impact
- Q1 2020 business net income increased 15.9% to €2,042 million and 16.1% at CER.
- Q1 2020 business EPS(2) was €1.63, up 15.6% at CER, with roughly half of this growth due to COVID-19 impact.
- IFRS EPS was €1.35 (up 48.4%).
R&D advances and regulatory milestones
- Detailed phase 2 results for BTK inhibitor (‘168) in multiple sclerosis presented at virtual scientific forum.
- Positive phase 3 results evaluating Dupixent® in children (6-11 years) with severe AD presented at RAD Virtual Conference.
- Sarclisa® approved in the U.S. for relapsed refractory multiple myeloma and favorable CHMP opinion received.
Full-year 2020 business EPS(2) guidance affirmed
- Sanofi continues to expect 2020 business EPS(2) to grow around 5%(3) at CER, barring unforeseen major adverse events. Sanofi expects the favorable first-quarter COVID-19 impact on sales and business EPS to be mainly offset during the second quarter. Applying average April 2020 exchange rates, the currency impact on 2020 business EPS is estimated to be between -1% to -2%.
Sanofi Chief Executive Officer, Paul Hudson, commented:
“I am proud of the way Sanofi employees responded to the immense challenges of the COVID-19 pandemic. They continue to put patients first while embracing and delivering on the new Company strategy. This is exemplified by the multi-pronged approach to fight COVID-19 with the accelerated development of vaccine candidates and therapeutics while sustaining the impressive growth of Dupixent®, the strength of the Vaccines business as well as driving efficiencies and cash flow. In R&D, we took actions to maintain clinical trial programs and to advance our pipeline of potentially transformative medicines. While the duration of the pandemic remains unknown at this point, I am confident Sanofi is well positioned to navigate these challenges and deliver on our commitment to patients.”
| Q1 2020 | Change | Change at CER | |
| IFRS net sales reported | €8,973m | +6.9% | +6.6% |
| IFRS net income reported | €1,683m | +48.0% | - |
| IFRS EPS reported | €1.35 | +48.4% | - |
| Free cash flow(4) | €1,558m | +90.0% | - |
| Business operating income | €2,659m | +15.8% | +15.9% |
| Business net income(2) | €2,042m | +15.9% | +16.1% |
| Business EPS(2) | €1.63 | +15.6% | +15.6% |
(1) Changes in net sales are expressed at constant exchange rates (CER) unless otherwise indicated (definition in Appendix 9); (2) In order to facilitate an understanding of operational performance, Sanofi comments on the business net income statement. Business net income is a non-GAAP financial measure (definition in Appendix 9). The consolidated income statement for Q1 2020 is provided in Appendix 3 and a reconciliation of reported IFRS net income to business net income is set forth in Appendix 4; (3) Base for business EPS growth is €5.97, reflecting 2 cents impact from IFRS 16 (Appendix 9); (4) Free cash flow is a non-GAAP financial measure (definition in Appendix 9).
R&D update
Consult Appendix 7 for full overview of Sanofi’s R&D pipeline
Regulatory update
Regulatory updates since February 6, 2020 include the following:
- In April, Efluelda® (Quadrivalent Influenza Vaccine-High Dose) obtained a positive end of the European procedure (decentralized procedure) for active immunization in adults aged 65 years of age and older for the prevention of influenza disease, allowing national licenses to be issued.
- In March, Sarclisa® (isatixumab-irfc) was approved by the U.S. Food and Drug Administration (FDA) in combination with pomalidomide and dexamethasone (pom-dex) for the treatment of adults with relapsed refractory multiple myeloma (RRMM) who have received at least two prior therapies including lenalidomide and a proteasome inhibitor. At the end of March, the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) also adopted a positive opinion for Sarclisa® in combination with pom-dex for the treatment of adult RRMM who have received at least two prior therapies.
At the end of April 2020, the R&D pipeline contained 87 projects, including 35 new molecular entities in clinical development (or that have been submitted to the regulatory authorities). 39 projects are in phase 3 or have been submitted to the regulatory authorities for approval.
Portfolio update
Phase 3:
- Pivotal phase 3 results evaluating Dupixent® (dupilumab) combined with standard-of-care topical corticosteroids (TCS) in children aged 6-11 years with uncontrolled severe atopic dermatitis were presented during a session at the 2020 Revolutionizing Atopic Dermatitis (RAD) Virtual Conference on April 5. These results demonstrated that Dupixent® combined with standard-of-care TCS significantly improved disease signs, symptoms and health-related quality of life. Sanofi and Regeneron previously announced positive topline results from this trial in August 2019.
- Sanofi and Regeneron Initiated a global clinical program evaluating Kevzara® (sarilumab) in patients hospitalized with severe COVID-19. Kevzara® inhibits IL-6, which may play a role in driving the inflammatory immune response that causes acute respiratory distress syndrome observed in patients with severe COVID-19 infection. Sanofi is leading trials outside the U.S., while Regeneron is leading U.S. trials.
- Two studies were initiated to evaluate Plaquenil® (hydroxychloroquine) as a potential treatment for COVID-19.
- A phase 3 study initiated to evaluate venglustat (GZ402671), an oral GCS Inhibitor, in the treatment of GM2 gangliosidosis, is pending the first patient enrollment.
- A phase 3 study initiated to evaluate Sarclisa® (isatuximab), in the treatment of smoldering multiple myeloma, is pending the first patient enrollment.
Phase 2
- A phase 2 study evaluating Sarclisa® (isatuximab-irfc) was initiated in patients awaiting kidney transplantation.
- The development of SAR440340 (collaboration with Regeneron), an anti-IL33 monoclonal antibody, in atopic dermatitis was discontinued due to lack of efficacy.
Collaboration
- On April 14, Sanofi and GSK announced that they had signed a letter of intent to develop an adjuvanted vaccine for COVID-19, using innovative technology from both companies, to help address the ongoing pandemic. Sanofi will contribute its S-protein COVID-19 antigen, which is based on recombinant DNA technology. GSK will contribute its proven pandemic adjuvant technology.
- On March 27, Sanofi announced a collaboration with Translate Bio, a clinical-stage messenger RNA (mRNA) therapeutics company, to develop a novel mRNA vaccine for COVID-19. This collaboration leverages an existing agreement from 2018 between the two companies to develop mRNA vaccines for infectious diseases.
- On February 18, Sanofi announced a collaboration with the Biomedical Advanced Research and Development Authority (BARDA). Sanofi Pasteur, will use its recombinant DNA platform and leverage previous development work for a SARS vaccine which may unlock a fast path forward for developing a novel COVID-19 vaccine.
- On April 16, Sanofi and Luminostics announced that they had signed an agreement to evaluate a collaboration on a unique self-testing solution for COVID-19, using Luminostics innovative technology, and further adding to Sanofi’s ongoing efforts to fight the COVID-19 pandemic on multiple fronts.
2020 first-quarter financial results(6)
Business Net Income(6)
In the first quarter of 2020, Sanofi generated net sales of €8,973 million, an increase of 6.9% and 6.6% at CER. About half of sales growth at CER was due to the net impact of COVID-19.
First-quarter other revenues increased 6.5% (up 3.4% at CER) to €343 million, including the VaxServe sales contribution of non-Sanofi products (€286 million, up 14.9% at CER).
First-quarter Gross Profit increased 6.1% to €6,469 million (up 5.5% at CER). The gross margin ratio decreased 0.6 percentage points to 72.1% (71.9% at CER) versus the first quarter of 2019. The negative impact from net price adjustments of Plavix® and the Aprovel® family in China and U.S. Diabetes net price evolution more than offset the favorable impact from Specialty Care growth and industrial productivity. In the first quarter of 2020, the gross margin ratio of segments were 74.8% for Pharmaceuticals (down 1.1 percentage points), 64.6% for Vaccines (up 2.3 percentage points) and 67.9% for CHC (down 1.3 percentage points).
Research and Development (R&D) expenses decreased 3.2% to €1,340 million in the first quarter of 2020. At CER, R&D expenses decreased 4.3% reflecting smart spending initiatives as well as a decline in Diabetes R&D expenses. In the first quarter, the ratio of R&D to sales decreased 1.6 percentage points to 14.9% compared to the first quarter of 2019.
First-quarter selling general and administrative expenses (SG&A) decreased 1.4% to €2,342 million. At CER, SG&A expenses were down 2.1%, reflecting smart spending initiatives. In the first quarter, the ratio of SG&A to sales decreased 2.2 percentage points to 26.1% compared to the first quarter of 2019.
First-quarter operating expenses were €3,682 million, a decrease of 2.1% and 2.9% at CER.
First-quarter other current operating income net of expenses was -€247 million versus -€102 million in the first quarter of 2019. In the first quarter of 2020, this line included an expense of €243 million (versus a €75 million expense in the first quarter of 2019) corresponding to the share of profit to Regeneron of the monoclonal antibodies Alliance, reimbursement of development costs by Regeneron and the reimbursement of commercialization-related expenses incurred by Regeneron.
The share of profit from associates was €131 million in the first quarter versus €71 million in the first quarter of 2019. The majority of the increase was due to discrete items in the equity accounting treatment of Sanofi’s ownership in Regeneron, including adjustments related to IFRS versus U.S. GAAP and prior period true-up based on actual reported results.
In the first quarter, non-controlling interests were -€12 million versus -€10 million in prior period.
First-quarter business operating income (BOI) increased 15.8% to €2,659 million. At CER, BOI increased 15.9%. The ratio of BOI to net sales increased 2.2 percentage points to 29.6% versus the first quarter of 2019. Over the period, the BOI ratio of segments were 39.4% for Pharmaceuticals (up 2.5 percentage points), 26.8% for Vaccines (down 0.2 percentage points) and 37.1% for CHC (up 0.2 percentage points).
Net financial expenses were -€75 million in the first quarter versus -€54 million in the same period of 2019. The first quarter of 2019 included a €26 million financial gain in connection with contingent payments on future regulatory milestones.
First-quarter effective tax rate was stable at 22.0% versus the prior period. Sanofi continues to expect its effective tax rate to be around 22% in 2020.
First-quarter business net income(6) increased 15.9% to €2,042 million and increased 16.1% at CER. The ratio of business net income to net sales increased 1.8 percentage points to 22.8% versus the first quarter of 2019.
In the first quarter of 2020, business earnings per share(6) (EPS) increased 15.6% to €1.63 on both a reported basis and at CER, with roughly half of this growth due to the COVID-19 impact. The average number of shares outstanding was 1,251.3 million versus 1,245.8 million in the first quarter of 2019.
(6) See Appendix 3 for 2020 first-quarter consolidated income statement; see Appendix 9 for definitions of financial indicators, and Appendix 4 for reconciliation of IFRS net income reported to business net income.
Reconciliation of IFRS net income reported to business net income (see Appendix 4)
In the first quarter of 2020, the IFRS net income was €1,683 million. The main items excluded from the business net income were:
- An amortization charge of €457 million related to fair value remeasurement on intangible assets of acquired companies (primarily Genzyme: €162 million, Bioverativ: €85 million, Boehringer Ingelheim CHC business: €51 million, Aventis: €35 million) and to acquired intangible assets (licenses/products: €22 million). These items have no cash impact on the Company.
- An impairment of intangible assets of €85 million mainly related to discontinued Diabetes projects.
- Restructuring costs and similar items of €66 million.
- A pre-tax gain of €108 million arising from the divestment of Seprafilm to Baxter.
- A €108 million tax effect arising from the items listed above, mainly comprising €142 million of deferred taxes generated by amortization and impairments of intangible assets and €20 million associated with restructuring costs and similar items. (see Appendix 4).
- An income of €27 million net of tax related to restructuring costs of associates and joint ventures and expenses arising from the impact of acquisitions on associates and joint ventures.
Capital Allocation
In the first quarter of 2020, free cash flow(7) increased by 90.0% to €1,558 million, after net changes in working capital (-€414 million), capital expenditures (-€319 million) and other asset acquisitions1 (-€165 million) net of disposal proceeds1 (€448 million), and payments related to restructuring and similar items (-€277 million). Over the period, acquisitions2 were €2,245 million (related to Synthorx). As a consequence, net debt increased from €15,107 million at December 31, 2019, to €16,191 million at March 31, 2020 (amount net of €7,279 million cash and cash equivalents).
1 Not exceeding €500 million per transaction.
2 Amount of the transaction above €500 million per transaction.
(7) non-GAAP financial measure (definition in Appendix 9).
To access the full press release of the 2020 Q1 results, please click here.
Forward-Looking Statements
This press release contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. These statements include projections and estimates and their underlying assumptions, statements regarding plans, objectives, intentions and expectations with respect to future financial results, events, operations, services, product development and potential, and statements regarding future performance. Forward-looking statements are generally identified by the words “expects”, “anticipates”, “believes”, “intends”, “estimates”, “plans” and similar expressions. Although Sanofi’s management believes that the expectations reflected in such forward-looking statements are reasonable, investors are cautioned that forward-looking information and statements are subject to various risks and uncertainties, many of which are difficult to predict and generally beyond the control of Sanofi, that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include among other things, the uncertainties inherent in research and development, future clinical data and analysis, including post marketing, decisions by regulatory authorities, such as the FDA or the EMA, regarding whether and when to approve any drug, device or biological application that may be filed for any such product candidates as well as their decisions regarding labelling and other matters that could affect the availability or commercial potential of such product candidates, the fact that product candidates if approved may not be commercially successful, the future approval and commercial success of therapeutic alternatives, Sanofi’s ability to benefit from external growth opportunities, to complete related transactions and/or obtain regulatory clearances, risks associated with intellectual property and any related pending or future litigation and the ultimate outcome of such litigation, trends in exchange rates and prevailing interest rates, volatile economic and market conditions, cost containment initiatives and subsequent changes thereto, and the impact that COVID-19 will have on us, our customers, suppliers, vendors, and other business partners, and the financial condition of any one of them, as well as on our employees and on the global economy as a whole. Any material effect of COVID-19 on any of the foregoing could also adversely impact us. This situation is changing rapidly and additional impacts may arise of which we are not currently aware and may exacerbate other previously identified risks. The risks and uncertainties also include the uncertainties discussed or identified in the public filings with the SEC and the AMF made by Sanofi, including those listed under “Risk Factors” and “Cautionary Statement Regarding Forward-Looking Statements” in Sanofi’s annual report on Form 20-F for the year ended December 31, 2019. Other than as required by applicable law, Sanofi does not undertake any obligation to update or revise any forward-looking information or statements.


