Vascepa® (icosapent ethyl) Showed 30% Reduction in Total Cardiovascular Events Including Recurrent Events in REDUCE-IT™
Approximately 159 Fewer Major Adverse Cardiovascular Events per 1000 Patients Studied
Data Show Relative Benefits of Vascepa Usage Grow Over Time
Amarin Schedules Webcast Discussion of Presented Data Today, March 18, 2019 at 4:00-5:00 pm CT
BEDMINSTER, N.J., and DUBLIN, Ireland, March 18, 2019 -- Amarin Corporation plc (NASDAQ:AMRN), presented new data from its landmark cardiovascular outcomes study of its prescription therapy, Vascepa® (icosapent ethyl), the REDUCE-IT™ study, showing that Vascepa provided a statistically significant 30% risk reduction in total (first and subsequent) cardiovascular events compared to placebo in the statin-treated patient population studied in REDUCE-IT. These data presented today as a late-breaker presentation at the American College of Cardiology’s (ACC) 68th Annual Scientific Session in New Orleans, LA, and published simultaneously in the Journal of the American College of Cardiology, extend the scope of consistent effects of Vascepa beyond a patient’s first cardiovascular event to all subsequent cardiovascular events, including cardiovascular death.1
In November 2018, groundbreaking primary results of the REDUCE-IT study were presented and published showing that Vascepa achieved the primary endpoint of the study demonstrating a statistically significant 25% placebo-controlled risk reduction in the first occurrence of major adverse cardiovascular events (MACE) as well as statistically significant relative risk reductions in each component of the MACE composite, consisting of cardiovascular death, heart attack, stroke, coronary revascularization and hospitalization for unstable angina. For the primary endpoint, a clinically impactful number needed to treat of 21 was reported.
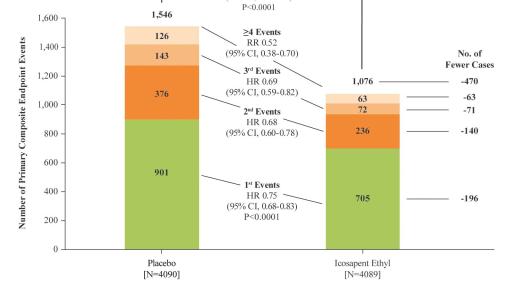
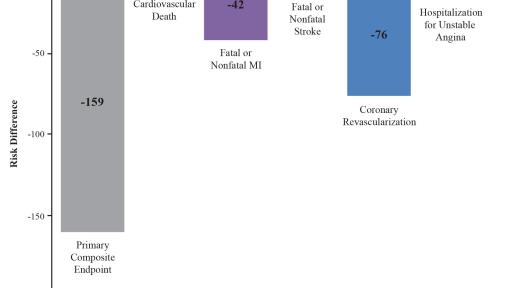
In the newly reported data, investigators led by the study’s principal investigator, Deepak L. Bhatt, MD, MPH, Professor of Medicine at Harvard Medical School, Executive Director of Interventional Cardiovascular Programs in the Heart and Vascular Center at Brigham and Women’s Hospital, and the Principal Investigator and Steering Committee Chair for REDUCE-IT, evaluated patients’ total cardiovascular events during the median study follow up of 4.9 years in REDUCE-IT. These analyses were tertiary or exploratory endpoints in REDUCE-IT. Total events included both a patient’s first occurrence of MACE as well as all subsequent occurrences of MACE. Recurrent cardiovascular events are common in people who have already had a heart attack. Various studies have found a recurrence rate of close to 50% for any cardiovascular event or for subsequent coronary revascularization in the year after a heart attack, and up to 75% of patients have a recurrent event within 3 years.2 Vascepa reduced total events, first and subsequent events, by 30% compared to placebo, reflecting that for every 1000 patients treated for 5 years with icosapent ethyl versus placebo approximately 159 MACE could be prevented with Vascepa, including prevention of approximately 12 cardiovascular deaths, 42 heart attacks (myocardial infarctions), 14 strokes, 76 coronary revascularizations and 16 episodes of hospitalization for unstable angina. There was also a 28% reduction of total events in the key secondary endpoint of 3-point MACE in the intent-to-treat population consisting of a composite of cardiovascular death, nonfatal heart attack and nonfatal stroke.
“This is an impressive degree of risk reduction,” said Dr. Bhatt. “From a patient’s perspective — and from my perspective as a physician — we care about repeat events and the risk of surviving a first stroke or heart attack only to go on to have a subsequent, and potentially fatal, event. The degree of benefit that this analysis reveals is quite large, especially considering that this is an additional layer of benefit on top of what statin and other therapies have already provided.”
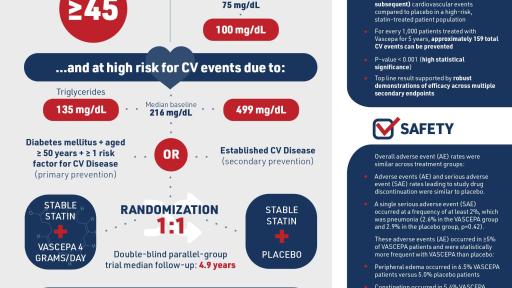
REDUCE-IT was a global study of 8,179 patients who, despite stable statin therapy, had elevated triglyceride levels (at least 135 mg/dL) and either documented cardiovascular disease or diabetes with other cardiovascular risk factors. Many patients with well-managed LDL-cholesterol remain at high risk for cardiovascular events. No therapy is currently approved to treat such residual cardiovascular risk in the population studied in REDUCE-IT and, prior to the successful results of Vascepa demonstrated in the study, no other therapy had demonstrated a 25% risk reduction compared to placebo on top of statin therapy in a major cardiovascular outcomes trial within the primary endpoint of any patient population. REDUCE-IT studied Vascepa 4 grams/day as compared to placebo.
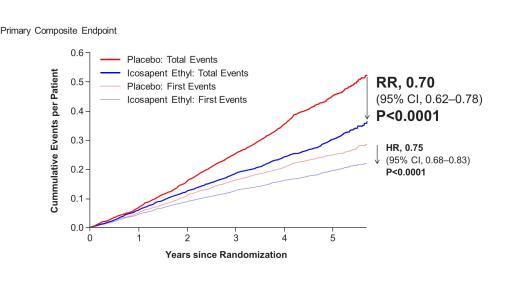
Benefits of Vascepa with respect to total cardiovascular event reduction were shown to continue over time as displayed below in the cumulative event curves of study results. The cardiovascular event curve for Vascepa visually separated from the placebo event curve at approximately year one and continued to separate throughout the remaining follow-up period. This relatively early and continued separation of total cardiovascular event rates is consistent with the primary events data (i.e., first occurrence data) from REDUCE-IT previously reported. The separation was significant with respect to the primary endpoint of first events and grows further over time for total cardiovascular events.
Figure 1: Total (First and Subsequent) Event Curves and Time to First Primary Composite Endpoint Curves
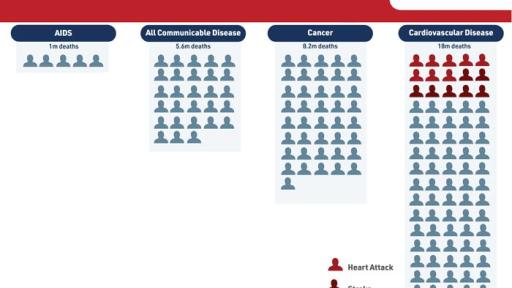
The relative risk reduction demonstrated by Vascepa in REDUCE-IT has implications for both patient health and the cost of healthcare. Cardiovascular disease is the number one cause of death in the United States and most of the world. In the U.S., there is one cardiovascular death every 38 seconds. Cardiovascular disease is the most expensive area of healthcare. Treating major adverse cardiovascular events is expensive both at the time of the event and often for years to follow. This cost is not only financial; it impacts patients through pain and suffering and loss of productivity. Preventing such cardiovascular events would be beneficial for patients, their families and for healthcare at-large. Amarin believes that reducing approximately 159 MACE per 1000 patients treated will position Vascepa well in pharmacoeconomic analyses planned to be conducted and reported in 2019.
Figure 2: Distribution of First, Subsequent and Total MACE Events for Patients Randomized 1:1 to Icosapent Ethyl Versus Placebo
Figure 3: Risk Differences (Number of Fewer Total Events Per 1000 Patients Treated for Median of 4.9 Years) with Icosapent Ethyl Versus Placebo for the Total Components of the Primary Composite Endpoint
No new safety related results from REDUCE-IT were reported with this new data. Safety data associated with REDUCE-IT was previously published in The New England Journal of Medicine3 and is provided below.
Commenting on this new data, John F. Thero, president and CEO of Amarin said “We believe that the robustly positive cardiovascular outcomes results demonstrated with Vascepa opens the door to a new era in preventative cardiovascular care which can potentially benefit millions of at-risk patients. Just as the REDUCE-IT results demonstrated that the effects of Vascepa are unprecedented in reducing cardiovascular risk in at-risk patients, as separately published, the mechanism of action of the unique small molecule, single active ingredient in Vascepa is multifactorial and differentiated from any other therapy.”
Dr. Steven Ketchum, president of research and development and chief scientific officer of Amarin stated, “We appreciate the ACC’s designation of the new results from REDUCE-IT as late-breaking clinical data as such designation reflects ACC’s recognition of the importance of the REDUCE-IT study and these potentially paradigm-changing results. As we witnessed with our two earlier successful Phase 3 studies of Vascepa, the MARINE and ANCHOR studies, we believe clinical results from Vascepa studies are robust and consistently favorable. We look forward to witnessing how these results improve patient care and to further evaluation and publication of data pertaining to Vascepa and REDUCE-IT. We remain very thankful to everyone involved in this landmark study.”
Amarin Investor/Analyst Conference Call
Amarin plans to webcast live a physician panel discussion for investors and analysts later today (Monday, March 18) at 4:00 p.m. Central Time (CT) / 5:00 p.m. Eastern Time (ET). During the panel discussion leading physicians are anticipated to review data pertaining to Vascepa presented at ACC’s 68th Annual Scientific Session, including the scheduled presentation today in the late-breaker session regarding additional data from the REDUCE-IT cardiovascular outcomes study. The panel discussion may also cover data from the above described poster and from other posters presented at ACC.
This physician panel discussion will commence at the time shown above and will be accessible via webcast through the investor relations section of the company’s website at www.amarincorp.com. The panel discussion can also be heard via telephone by dialing 877-407-8033. A replay of the panel discussion will be made available for a period of two weeks following the webcast. To hear a replay of the call, dial 877-481-4010 (inside the United States) or 919-882-2331 (outside the United States). A replay of the panel discussion will also be available through the company’s website shortly after the webcast. For both dial-in numbers please use conference ID 44518.
About Amarin
Amarin Corporation plc. is a rapidly growing, innovative pharmaceutical company focused on developing therapeutics to improve cardiovascular health. Amarin’s product development program leverages its extensive experience in polyunsaturated fatty acids and lipid science. Vascepa (icosapent ethyl) is Amarin’s first FDA-approved drug and is available by prescription in the United States, Lebanon and the United Arab Emirates. Amarin’s commercial partners are pursuing additional regulatory approvals for Vascepa in Canada, China and the Middle East. For more information about Amarin, visit www.amarincorp.com.
About REDUCE-IT
REDUCE-IT3, an 8,179-patient cardiovascular outcomes study, was completed in 2018. REDUCE-IT was the first multinational cardiovascular outcomes study that evaluated the effect of prescription pure EPA therapy as an add-on to statins in patients with high cardiovascular risk who, despite stable statin therapy, had elevated triglyceride levels (at least 135 mg/dL). A large portion of the male and female patients enrolled in this outcomes study were diagnosed with type 2 diabetes.
More information on the REDUCE-IT study results can be found at www.amarincorp.com.
About Cardiovascular Disease
Worldwide, cardiovascular disease (CVD) remains the #1 killer of men and women. In the United States CVD leads to one in every three deaths – one death approximately every 38 seconds – with annual treatment cost in excess of $500 billion.4, 5
Multiple primary and secondary prevention trials have shown a significant reduction of 25% to 35% in the risk of cardiovascular events with statin therapy, leaving significant persistent residual risk despite the achievement of target LDL-C levels.6
Beyond the cardiovascular risk associated with LDL-C, genetic, epidemiologic, clinical and real-world data suggest that patients with elevated triglycerides (TG) (fats in the blood), and TG-rich lipoproteins, are at increased risk for cardiovascular disease. 7, 8, 9, 10
About Vascepa (icosapent ethyl) Capsules
Vascepa (icosapent ethyl) capsules are a single-molecule prescription product consisting of the omega-3 acid commonly known as EPA in ethyl-ester form. Vascepa is not fish oil, but is derived from fish through a stringent and complex FDA-regulated manufacturing process designed to effectively eliminate impurities and isolate and protect the single molecule active ingredient from degradation. Vascepa, known in scientific literature as AMR101, has been designated a new chemical entity by the FDA. Amarin has been issued multiple patents internationally based on the unique clinical profile of Vascepa, including the drug’s ability to lower triglyceride levels in relevant patient populations without raising LDL-cholesterol levels.
Indication and Usage Based on Current FDA-Approved Label (not including REDUCE-IT results)
- Vascepa (icosapent ethyl) is indicated as an adjunct to diet to reduce triglyceride (TG) levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia.
- The effect of Vascepa on the risk for pancreatitis and cardiovascular mortality and morbidity in patients with severe hypertriglyceridemia has not been determined.
Important Safety Information for Vascepa Based on Current FDA-Approved Label (not including REDUCE-IT results) (Includes Data from Two 12-Week Studies (n=622) (MARINE and ANCHOR) of Patients with Triglycerides Values of 200 to 2000 mg/dL)
- Vascepa is contraindicated in patients with known hypersensitivity (e.g., anaphylactic reaction) to Vascepa or any of its components.
- In patients with hepatic impairment, monitor ALT and AST levels periodically during therapy.
- Use with caution in patients with known hypersensitivity to fish and/or shellfish.
- The most common reported adverse reaction (incidence >2% and greater than placebo) was arthralgia (2.3% for Vascepa, 1.0% for placebo). There was no reported adverse reaction >3% and greater than placebo.
- Adverse events and product complaints may be reported by calling 1-855-VASCEPA or the FDA at 1-800-FDA-1088.
- Patients receiving treatment with Vascepa and other drugs affecting coagulation (e.g., anti-platelet agents) should be monitored periodically.
- Patients should be advised to swallow Vascepa capsules whole; not to break open, crush, dissolve, or chew Vascepa.
FULL VASCEPA PRESCRIBING INFORMATION CAN BE FOUND AT WWW.VASCEPA.COM.
Important Safety Information for Vascepa based on REDUCE-IT, as previously reported in The New England Journal of Medicine3 publication of the primary results of the REDUCE-IT study:
- Excluding the major adverse cardiovascular events (MACE) results described above, overall adverse event rates in REDUCE-IT were similar across the statin plus Vascepa and the statin plus placebo treatment groups.
- There were no significant differences between treatments in the overall rate of treatment emergent adverse events or serious adverse events leading to withdrawal of study drug.
- There was no serious adverse event (SAE) occurring at a frequency of >2% which occurred at a numerically higher rate in the statin plus Vascepa treatment group than in the statin plus placebo treatment group.
- Adverse events (AEs) occurring in 5% or greater of patients and more frequently with Vascepa than placebo were:
- peripheral edema (6.5% Vascepa patients versus 5.0% placebo patients), although there was no increase in the rate of heart failure in Vascepa patients
- constipation (5.4% Vascepa patients versus 3.6% placebo patients), although mineral oil, as used as placebo, is known to lower constipation, and
- atrial fibrillation (5.3% Vascepa patients versus 3.9% placebo patients), although there were reductions in rates of cardiac arrest, sudden death and myocardial infarctions observed in Vascepa patients
- There were numerically more SAEs related to bleeding in the statin plus Vascepa treatment group although overall rates were low with no fatal bleeding observed in either group and no significant difference in adjudicated hemorrhagic stroke or serious central nervous system or gastrointestinal bleeding events between treatments.
- In summary, Vascepa was well tolerated with a safety profile generally consistent with clinical experience associated with omega-3 fatty acids and current FDA-approved labeling of such products.
Vascepa has been approved for use by the United States Food and Drug Administration (FDA) as an adjunct to diet to reduce triglyceride levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia. FDA has not reviewed and opined on a supplemental new drug application related to REDUCE-IT. FDA has not reviewed the information herein or determined whether to approve Vascepa for use to reduce the risk of MACE. Nothing in this press release should be construed as promoting the use of Vascepa in any indication that has not been approved by the FDA.
Important Cautionary Information About These Data
Recurrent event analyses for the total primary endpoint events and for the total key secondary endpoint in REDUCE-IT as published in the Journal of the American College of Cardiology and presented here were conducted using a series of statistical models. These analyses were tertiary or exploratory endpoints; most of the models used were prespecified and one was post hoc. Each recurrent event statistical model has inherent strengths and weaknesses, with no single model considered definitive or outperforming the other models, and this is an evolving field of science. Nonetheless, results from the total primary and total key secondary endpoint events analyses are consistent across the various recurrent event statistical models and are also consistent with the original primary and secondary endpoint results. Together, the REDUCE-IT recurrent event analyses and the original primary and key secondary endpoint analyses support the robustness of the clinical benefit of Vascepa therapy in reducing cardiovascular risk.
Further REDUCE-IT data assessment and data release could yield additional useful information to inform greater understanding of the trial outcome. Further detailed data assessment by Amarin and regulatory authorities will continue and take several months to complete and record. The final evaluation of the totality of the efficacy and safety data from REDUCE-IT may include some or all of the following, as well as other considerations: new information affecting the degree of treatment benefit on studied endpoints; study conduct and data robustness, quality, integrity and consistency; additional safety data considerations and risk/benefit considerations; consideration of REDUCE-IT results in the context of other clinical studies.
Forward-Looking Statements
This press release contains forward-looking statements, including expectations that REDUCE-IT results could lead to a new treatment paradigm in the patient population studied, help millions of patients, favorably affect the cost of treatment for cardiovascular disease and related productivity. These forward-looking statements are not promises or guarantees and involve substantial risks and uncertainties. In addition, Amarin’s ability to effectively commercialize Vascepa will depend in part on its ability to continue to effectively finance its business, efforts of third parties, its ability to gain regulatory approvals, create market demand for Vascepa through education, marketing and sales activities, to achieve market acceptance of Vascepa, to receive adequate levels of reimbursement from third-party payers, to develop and maintain a consistent source of commercial supply at a competitive price, to comply with legal and regulatory requirements in connection with the sale and promotion of Vascepa and to maintain patent protection for Vascepa. Among the factors that could cause actual results to differ materially from those described or projected herein include the following: uncertainties associated generally with research and development, clinical trials and related regulatory approvals; the risk that sales may not meet expectations and related cost may increase beyond expectations; the risk that patents may not be upheld in patent litigation and applications may not result in issued patents sufficient to protect the Vascepa franchise. A further list and description of these risks, uncertainties and other risks associated with an investment in Amarin can be found in Amarin’s filings with the U.S. Securities and Exchange Commission, including its most recent annual report on Form 10-K. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Amarin undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Availability of Other Information About Amarin
Investors and others should note that Amarin communicates with its investors and the public using the company website (www.amarincorp.com), the investor relations website (investor.amarincorp.com), including but not limited to investor presentations and investor FAQs, Securities and Exchange Commission filings, press releases, public conference calls and webcasts. The information that Amarin posts on these channels and websites could be deemed to be material information. As a result, Amarin encourages investors, the media, and others interested in Amarin to review the information that is posted on these channels, including the investor relations website, on a regular basis. This list of channels may be updated from time to time on Amarin’s investor relations website and may include social media channels. The contents of Amarin’s website or these channels, or any other website that may be accessed from its website or these channels, shall not be deemed incorporated by reference in any filing under the Securities Act of 1933.
References
1 Bhatt DL, Steg PG, Miller M, et al. Effects of Icosapent Ethyl on Total Ischemic Events – Further Insights from REDUCE-IT. J Am Coll Cardiol 2019. epub ahead of print.
http://www.onlinejacc.org/content/early/2019/03/01/j.jacc.2019.02.032
2 Bansilal S, Castellano JM, Fuster V. Global burden of CVD: focus on secondary prevention of cardiovascular disease. Int J Cardiol 2015;201:S1–S7.
3 Bhatt DL, Steg PG, Miller M, et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med 2019;380:11-22.
4 American Heart Association. 2018. Disease and Stroke Statistics-2018 Update.
5 American Heart Association. 2017. Cardiovascular disease: A costly burden for America projections through 2035.
6 Ganda OP, Bhatt DL, Mason RP, et al. Unmet need for adjunctive dyslipidemia therapy in hypertriglyceridemia management. J Am Coll Cardiol. 2018;72(3):330-343.
7 Budoff M. Triglycerides and triglyceride-rich lipoproteins in the causal pathway of cardiovascular disease. Am J Cardiol. 2016;118:138-145.
8 Toth PP, Granowitz C, Hull M, et al. High triglycerides are associated with increased cardiovascular events, medical costs, and resource use: A real-world administrative claims analysis of statin-treated patients with high residual cardiovascular risk. J Am Heart Assoc. 2018;7(15):e008740.
9 Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease - New insights from epidemiology, genetics, and biology. Circ Res. 2016;118:547-563.
10 Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384:626–635.
Amarin Contact Information
Investor Relations:
Elisabeth Schwartz
Investor Relations and Corporate Communications
Amarin Corporation plc
In U.S.: +1 (908) 719-1315
[email protected] (investor inquiries)
[email protected] (media inquiries)
Lee M. Stern
Trout Group
In U.S.: +1 (646) 378-2992
[email protected]