USP Announces Generics Access Plan
Quality standards can help increase the number of generic medicines available to patients
Rockville, Md., January 31, 2019 — USP today announced its Generics Access Plan, helping to increase access to medicines by facilitating generics competition through new and revised quality standards and related activities, including collaborations with the U.S. Food and Drug Administration (FDA) and others. USP’s new Generics Access Plan supports the FDA in its efforts to encourage development of new generic medicines to promote competition, help reduce drug prices and improve access to medicine for Americans.
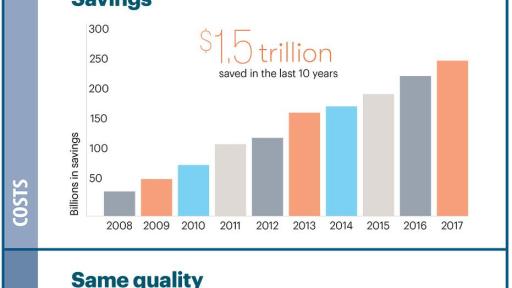
When generic medicines are available, they help save money: $1.5 trillion over the past 10 years. Yet today many off-patent meds have few or no generic options. Learn how the @uspharmacopeia #Generics Access Plan can help Tweet
USP, a scientific non-profit, develops public quality standards which apply to drug products sold in the U.S. to help ensure we can trust the quality of medicines. These standards provide generics manufacturers around the world with publically available requirements and testing methods, as well as expectations for quality, which can help them develop generic versions of off-patent drugs.
“Millions of Americans have benefitted from generic medicines and in just the last 10 years $1.5 trillion have been saved, yet there are still many off-patent medications that have few or no generic alternatives,” said Ronald T. Piervincenzi, Ph.D., USP chief executive officer. “While there has been much progress, there are still many off-patent drugs that have limited or no generic options. We must close this gap, which is why USP is committed to doing our part by developing new public standards and processes, working with the FDA and others to facilitate greater access to important drug therapies.”
USP’s standards have helped drug manufacturers develop generic versions of off-patent drugs. Many of these standards apply to drugs used to treat chronic health conditions like high blood pressure, high cholesterol, seizure disorders, mental health conditions and other major health concerns. Without public standards in place, it could be more difficult for some manufacturers to bring generics to market.
“Manufacturers can rely on USP quality standards, helping accelerate availability of generic versions of off-patent medicines,” said Piervincenzi. “This helps provide patients more access to affordable alternatives of the medicines they need.”
USP’s Generics Access Plan can help increase patient access to medicines by facilitating competition through new and revised standards, training and education, and collaborations with the FDA, industry representatives and others. The plan includes:
- Developing and updating quality standards supporting FDA’s Drug Competition Action Plan (DCAP) priorities, including FDA’s list of off-patent drugs for which generic alternatives are not available on the market. USP is prioritizing development of new public quality standards to help facilitate generic medicines in a number of therapeutic areas, including for medicines used to treat hypertension, seizure disorders, HIV and cancer.
- Offering training and education for generics manufacturers worldwide on best practices for applying quality standards to enhance the capabilities for producing quality generics
- USP supports generics manufacturers, regulators and other stakeholders around the world with education and best practices for using quality standards
- Examples include our (USP) Education programs, the USP Hyderabad Training Institute, and the USP-APEC Center of Excellence
- Convening regulators, industry representatives, patient groups, payers, healthcare practitioners and others to identify additional ways to support generics development through the standard-setting process and educational programming
USP develops its public standards through a collaborative process, working with independent experts from healthcare, academia, industry, and representatives from the FDA. “Our process establishing science-based quality standards for medicines is an essential component of the safety net that keeps our nation’s drug supply and patients safe,” said Jaap Venema, Ph.D., USP’s chief science officer.
About USP
USP is an independent scientific organization that collaborates with the world’s top experts in health and science to develop quality standards for medicines, dietary supplements, and food ingredients. Through our standards, advocacy and education, USP helps increase the availability of quality medicines, supplements and food for billions of people worldwide. Learn more at usp.org.
Media Contact Anne Bell, 301-998-6785 [email protected]