New Phase 3 data demonstrate superiority of TREMFYA® (guselkumab) vs Cosentyx® (secukinumab) in delivering PASI 90 responses in the treatment of moderate to severe plaque psoriasis at week 48
84.5 percent of patients receiving TREMFYA achieved the primary endpoint of a PASI 90 response at week 48 compared with 70.0 percent of patients receiving Cosentyx
ECLIPSE is the first Phase 3 head-to-head study to compare efficacy between the first-in-class IL-23 inhibitor, TREMFYA, and the IL-17 inhibitor, Cosentyx
HORSHAM, PENNSYLVANIA, December 12, 2018 - The Janssen Pharmaceutical Companies of Johnson & Johnson today announced results from the ECLIPSE study demonstrating that TREMFYA® (guselkumab) was superior to Cosentyx® (secukinumab)* in treating adults with moderate to severe plaque psoriasis for the primary endpoint assessed at week 48. Data from the multi-center, randomized, double-blind head-to-head Phase 3 study demonstrated that 84.5 percent of patients treated with TREMFYA achieved at least 90 percent improvement in their baseline Psoriasis Area Severity Index (PASI) score at week 48, compared with 70.0 percent of patients treated with Cosentyx (p<0.001).
These data, to be presented at the 3rd Inflammatory Skin Disease Summit in Vienna, December 12-15, mark the first-ever results from a head-to-head study comparing an interleukin (IL)-23-targeted biologic therapy (TREMFYA) with an IL-17 inhibitor (Cosentyx). ECLIPSE is Janssen’s fourth TREMFYA Phase 3 study in plaque psoriasis and is part of a comprehensive clinical development program that also includes ongoing Phase 3 studies in psoriatic arthritis and Crohn’s disease.
ECLIPSE incorporated six major secondary endpoints that used a fixed statistical sequence procedure to control for multiple comparisons and included both shorter and longer-term analyses. TREMFYA demonstrated non-inferiority to Cosentyx in the first major secondary endpoint, with 84.6 percent of patients on TREMFYA achieving a PASI 75 response at both weeks 12 and 48 vs. 80.2 percent of those on Cosentyx (p<0.001), however, it did not demonstrate superiority (p=0.062). Because superiority was not demonstrated for the first major secondary endpoint, p-values for all the subsequent major secondary endpoints were considered nominal.
Three of the remaining major secondary endpoints evaluated efficacy at week 48, including achievement of a PASI 100 response and Investigator’s Global Assessment (IGA) scores of 0 (cleared), or 0 or 1 (cleared or minimal disease). At week 48, 58.2 percent of patients receiving TREMFYA achieved a PASI 100 response, compared with 48.4 percent of patients receiving Cosentyx; 62.2 percent of patients receiving TREMFYA achieved an IGA score of 0 compared to 50.4 percent of patients receiving Cosentyx; and 85.0 percent of patients receiving TREMFYA achieved an IGA score of 0 or 1 compared to 74.9 percent of patients receiving Cosentyx (all comparisons with nominal p≤ 0.001).
The remaining major secondary endpoints assessed non-inferiority of TREMFYA versus Cosentyx at week 12. The percentage of patients achieving a PASI 75 response at week 12 was 89.3 percent for TREMFYA and 91.6 percent for Cosentyx (p<0.001 for non-inferiority); the percentage of patients achieving a PASI 90 response at week 12 was 69.1 percent for TREMFYA and 76.1 percent for Cosentyx (p=0.127 for non-inferiority).
“The response-over-time curves show that maximum response rates with TREMFYA are achieved after six months and are maintained over time through one year, achieving superiority at the primary endpoint of the study,” said lead study investigator Richard Langley, M.D., FRCPC, Professor, Division of Clinical Dermatology & Cutaneous Science, Department of Medicine, Dalhousie University. “Results of the study confirm a slightly more rapid onset of response with Cosentyx, but importantly, in a chronic disease like psoriasis, these data provide new insights into comparative longer-term efficacy.”
Through week 44, 27 patients (5.1 percent) randomized to the TREMFYA arm discontinued treatment compared with 48 patients (9.3 percent) randomized to the Cosentyx arm.
The safety profiles observed for TREMFYA and Cosentyx in ECLIPSE were consistent with the known safety profiles seen in the respective registration trials and current prescribing information. Similar percentages of patients receiving TREMFYA (77.9 percent), and Cosentyx (81.6 percent) reported at least one adverse event (AE). Serious AEs were reported in 6.2 percent of patients receiving TREMFYA and 7.2 percent of patients receiving Cosentyx. Serious infections occurred in six patients receiving TREMFYA and five patients receiving Cosentyx.
“Fortunately for patients, there are many good treatment options available for plaque psoriasis today. However, to make the best recommendation for their patients from among these options, physicians need long term comparative safety and efficacy data. We’re proud to have conducted this important trial to help guide clinical practice and continue to build on the robust database of clinical information that we’ve been able to generate on TREMFYA, the first IL-23 inhibitor,” said Newman Yeilding, M.D., Head of Immunology Development, Janssen Research & Development, LLC.
About ECLIPSE
The Phase 3, multicenter, randomized, double-blind, active comparator trial, ECLIPSE, was designed to evaluate the efficacy and safety of TREMFYA compared with Cosentyx in adult patients with moderate to severe plaque psoriasis. Patients (n=1048) were randomized to receive 100 mg of TREMFYA administered by subcutaneous injection at weeks 0, 4 and 12, followed by every eight-week dosing; or 300 mg of Cosentyx administered by two subcutaneous injections of 150 mg at weeks 0, 1, 2, 3, 4 followed by every 4-week dosing. The primary endpoint of the study was the proportion of patients achieving a PASI 90 response at week 48. Secondary endpoints were assessed at weeks 12 and 48, with safety monitoring through week 56.
About Psoriasis
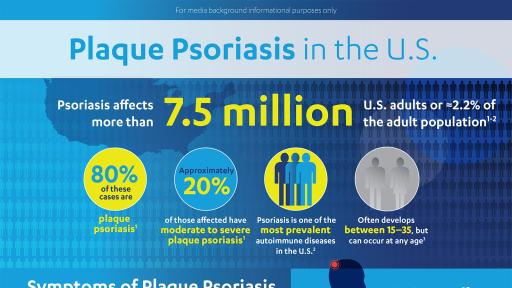
Psoriasis is a chronic, autoimmune inflammatory disorder that results in the overproduction of skin cells, characterized by raised, inflamed, red lesions, or plaques, which can cause physical pain and itching.1 It is estimated that more than 7.5 million Americans live with the disease.2 Approximately 80 percent of those affected with psoriasis have mild to moderate disease, while 20 percent have moderate to severe plaque psoriasis.1
About TREMFYA® (guselkumab)
TREMFYA® is a human monoclonal antibody developed by Janssen that selectively blocks the protein interleukin (IL)-23 and is approved in the U.S., Canada, European Union, Japan and a number of other countries worldwide for the treatment of adult patients with moderate to severe plaque psoriasis who may benefit from taking injections or pills (systemic therapy) or phototherapy (treatment using ultraviolet or UV light). Ongoing trials include: Two Phase 3 programs evaluating TREMFYA in the treatment of active psoriatic arthritis and a Phase 3 program in Crohn’s disease.
The Janssen Pharmaceutical Companies of Johnson & Johnson maintain exclusive worldwide marketing rights to TREMFYA®.
What is the most important information I should know about TREMFYA®? TREMFYA® may cause serious side effects, including infections. TREMFYA® is a prescription medicine that may lower the ability of your immune system to fight infections and may increase your risk of infections. Your healthcare provider should check you for infections and tuberculosis (TB) before starting treatment with TREMFYA® and may treat you for TB before you begin treatment with TREMFYA® if you have a history of TB or have active TB. Your healthcare provider should watch you closely for signs and symptoms of TB during and after treatment with TREMFYA®.
- fever, sweats, or chills
- muscle aches
- weight loss
- cough
- warm, red, or painful skin or sores on your body different from your psoriasis
- diarrhea or stomach pain
- shortness of breath
- blood in your phlegm (mucus)
- burning when you urinate or urinating more often than normal
Before using TREMFYA®, tell your healthcare provider about all of your medical conditions, including if you:
- have any of the conditions or symptoms listed in the section “What is the most important information I should know about TREMFYA®?”
- have an infection that does not go away or that keeps coming back.
- have TB or have been in close contact with someone with TB.
- have recently received or are scheduled to receive an immunization (vaccine). You should avoid receiving live vaccines during treatment with TREMFYA®.
- are pregnant or plan to become pregnant. It is not known if TREMFYA® can harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if TREMFYA® passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
What are the possible side effects of TREMFYA®?
TREMFYA® may cause serious side effects. See “What is the most important information I should know about TREMFYA®?”
The most common side effects of TREMFYA® include: upper respiratory infections, headache, injection site reactions, joint pain (arthralgia), diarrhea, stomach flu (gastroenteritis), fungal skin infections, and herpes simplex infections. These are not all the possible side effects of TREMFYA®. Call your doctor for medical advice about side effects.
Use TREMFYA® exactly as your healthcare provider tells you to use it.
Please read the full Prescribing Information, including Medication Guide for TREMFYA®, and discuss any questions that you have with your doctor.
You are encouraged to report negative side effects of prescription drugs to the FDA.
Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
About the Janssen Pharmaceutical Companies of Johnson & Johnson
At the Janssen Pharmaceutical Companies of Johnson & Johnson, we are working to create a world without disease. Transforming lives by finding new and better ways to prevent, intercept, treat and cure disease inspires us. We bring together the best minds and pursue the most promising science. We are Janssen. We collaborate with the world for the health of everyone in it. Learn more at http://www.janssen.com/. Follow us at http://www.twitter.com/JanssenGlobal. Janssen Research & Development, LLC is part of the Janssen Pharmaceutical Companies of Johnson & Johnson.
*Cosentyx is a trademark of Novartis AG.
*Dr. Langley is a paid consultant for Janssen. He was not compensated for any media work.
- American Academy of Dermatology. What is Psoriasis? https://www.aad.org/public/diseases/scaly-skin/psoriasis/what-is-psoriasis. Accessed November 19, 2018
- National Psoriasis Foundation. https://www.psoriasis.org/content/statistics. Accessed November 19, 2018
Media contacts:
Kellie McLaughlin
Mobile: (609) 468-8356
Megan Farina
Mobile: (610) 724-1079
Investor contacts:
Chris DelOrefice
Office: (732) 524-2955
Lesley Fishman
Office: (731) 524-3922