Dompé announces first treatment with Oxervate™ eye drops (cenegermin-bkbj), for neurotrophic keratitis (NK)
- Now available through Accredo, Oxervate is the first-in-class recombinant human nerve growth factor (rhNGF) and holds the potential to completely heal NK.
- Oxervate has the potential to resolve the rare and progressive eye disease with one eight-week treatment cycle.
- Dompé launches Dompé Connect to Care, comprehensive patient support programs to facilitate patient access.
MILAN, Italy and SAN BRUNO, Calif., January 3 2019. – Dompé Farmaceutici S.p.A and Dompé U.S. Inc., (collectively Dompé), announce the first treatment of a patient with Oxervate eye drops (cenegermin-bkbj), in the United States. Oxervate was approved by the U.S. Food and Drug Administration (FDA) in August 2018 as the first drug specifically indicated to treat neurotrophic keratitis (NK), a rare and progressive eye disease that can lead to corneal scarring and visual loss.1
“I am excited to be among the first physicians to treat neurotrophic keratitis patients with this new therapy,” said Giacomina Massaro-Giordano, MD, of the Scheie Eye Institute at the University of Pennsylvania and an investigator in the Oxervate clinical trials. “This newly approved therapy provides clinical improvements for NK patients, many of whom are at risk for losing their sight, and treatment options to help them were previously limited to symptom management.”
Patient Access to Oxervate
Prior to commercial availability, Oxervate was provided to individual patients through an Expanded Access Program with approval from the FDA. Dompé remains committed to patient access and has provided a comprehensive patient support program called Dompé Connect to Care (DC2C). DC2C will provide educational resources on NK and Oxervate for both patients and physicians. DC2C also provides financial assistance to eligible patients through the Oxervate Co-pay Card Program, and the Dompé Patient Assistance Program. More information on these programs is available by calling 1-833-DOMPE-US. Dompé is also a member of the Corporate Council of the National Organization for Rare Diseases (NORD) and supports the organization’s RareCare® program for patients with rare diseases, including neurotrophic keratitis.
“We would like to thank the patients and physicians who participated in our clinical trials and the ophthalmic and rare disease communities who have contributed to making Oxervate available,” said Sergio Dompé, Chairman of Dompé. “At Dompé our top priority is ensuring that NK patients are able to access the clinical benefits of Oxervate. We are also committed to continuing our investments in innovation, including our programs related to nerve growth factor and in other areas of unmet medical need.”
Oxervate is now commercially available in the U.S. and can be ordered through Accredo Specialty Pharmacy by calling 1-877-831-8112.
Oxervate is the first-ever FDA-approved application of a human nerve growth factor as drug or treatment, and the first-ever topical biologic medication approved in ophthalmology. Oxervate was approved in August 2018 as an orphan drug by the FDA after being granted Breakthrough Therapy Designation, Fast Track Status, and Priority Review. Oxervate is approved in multiple jurisdictions worldwide, including those in the European Union.
Oxervate: Safety and Efficacy Data
The efficacy and safety of Oxervate in NK was established in two independent, double-masked, randomized, multi-center, controlled clinical trials.2,3 This program represents the largest combined population of patients with NK ever examined in randomized controlled trials. Both trials studied Oxervate monotherapy (20 mcg/mL) as compared to vehicle, a proxy for preservative-free artificial tears, among patients with moderate or severe NK. Study NGF0212 (REPARO), which was conducted in Europe, randomized 52 patients to each group. After eight weeks, 72.0 percent of patients in the treatment group were completely healed vs 33.3 percent in the vehicle group. Study NGF0214, conducted in the U.S., randomized 24 patients to each group, and 65.2 percent of treated patients were completely healed vs 16.7 percent in the vehicle group. In REPARO, the study with the longer follow-up, approximately 80 percent of patients who healed during the eight-week treatment period remained healed after one year.4
Oxervate was well tolerated in clinical trials. Eye pain, which investigators reported can be associated with regaining sensation that has been lost due to the disease, was the most frequent adverse reaction observed with Oxervate, and was reported in approximately 16 percent of patients.4,5 Other adverse events reported in more than five percent of patients included corneal deposits, swelling (inflammation) of the eye, increase of tears (increased lacrimation) and ocular hyperemia.4 Findings from the REPARO trial program are published in the September 2018 issue of Ophthalmology, the journal of the American Academy of Ophthalmology. Topline data from the confirmatory NGF0214 study were presented at the 2017 Congress of the European Society of Ophthalmology, and trial results are in preparation for publication.
About Neurotrophic Keratitis
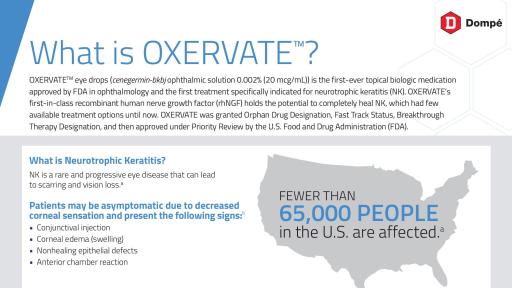
Neurotrophic keratitis, also known as neurotrophic keratopathy, is a rare disease that can be caused by damage to any level of the fifth cranial (trigeminal) nerve. Causes of impaired corneal sensation include herpetic or other infections, ocular or neurologic surgeries, physical injury to the ocular surface, and some systemic conditions that impair sensation. Neurotrophic keratitis can progress to corneal scarring and vision loss. Diagnosis, prognosis, and treatment are based on disease severity, which is classified broadly into three stages. Stage 1 (mild) exhibits ocular surface irregularity and reduced vision, stage 2 (moderate) exhibits a non-healing persistent epithelial defect (PED), and stage 3 (severe) exhibits corneal ulceration involving subepithelial (stromal) tissue, which may progress to corneal melting and perforation. Patients with the more advanced stages of neurotrophic keratitis are typically treated by cornea specialists. Neurotrophic keratitis affects fewer than 65,000 persons in the U.S. based on estimated disease prevalence.1
The cornea is the most densely innervated tissue in humans, with approximately 7,000 nerve endings per square millimeter. These nerves are unique in providing support to the cornea, which is crucial to help maintain transparency in this tissue that is normally devoid of blood vessels. In fact, corneal nerves help maintain ocular surface health by producing soluble factors called neurotrophins and facilitating sensory-dependent blinking and tearing reflexes.6
About Oxervate (Cenegermin-bkbj Ophthalmic Solution)
Oxervate is a topical solution 0.002% of cenegermin-bkbj (20 mcg/mL) administered six times per day for eight weeks to treat neurotrophic keratitis. Its active ingredient is a recombinant form of human nerve growth factor, a protein made by the human body. Nerve growth factor (NGF) acts through specific high-affinity and low-affinity nerve growth factor receptors in the anterior segment of the eye to support corneal innervation and integrity.
More information on Oxervate can be found here in the full prescribing information on the product: www.oxervate.com
Important Safety Information
The most common adverse reaction in clinical trials that occurred more frequently with Oxervate was eye pain (16% of patients). Other adverse reactions included corneal deposits, foreign body sensations in the eye, ocular hyperemia (enlarged blood vessels in the white of the eyes), swelling (inflammation) of the eye, and increase of tears (1-10% of patients).
About Dompé
Dompé is a private, rapidly scaling global biopharmaceutical company founded in Milan, Italy, with a 165-year legacy of medical innovation. Today, Dompé employs 700 employees worldwide and is growing in the United States, Spain, Germany and France. In the United States, the commercial operations hub is located in the San Francisco Bay Area, and the company maintains an R&D presence in Boston.
Forward Looking Statements
This press release makes reference to certain information that may not coincide with expected future results. Dompé firmly believes in the soundness and reasonableness of the concepts expressed. However, some of the information is subject to a certain degree of indetermination in relation to its research and development activities and the necessary verifications to be performed by regulatory bodies. Therefore, as of today, Dompé cannot guarantee that the expected results will be consistent with the information provided above.
Contacts
For the United States:
Christy Curran
(615) 414-8668
[email protected]
For Europe:
Benedetta Bitozzi
Mob. +39 3667742408
[email protected]
Stefano Amoroso
Tel. +39 02 58383275 – Mob. +39 340 2838136
[email protected]
1 Sacchetti M, Lambiase A. Diagnosis and Management of Neurotrophic Keratitis. Clinical Ophthalmology. 2014;8:571-9.
2 Bonini S, Lambiase A, Rama P, Sinigaglia F, Allegretti M, Chao W, Mantelli F, for the REPARO Study Group. Phase II Randomized, Double-Masked, Vehicle-Controlled Trial of Recombinant Human Nerve Growth Factor for Neurotrophic Keratitis. Ophthalmology. 2018;125:1332-43.
3 Chao W, Benitez Del Castillo JM, Dana R, Geerling G, Mantelli F, Massaro-Giordano G, Rama P. Healing of Persistent Epithelial Defects or Corneal Ulcer by Recombinant Human Nerve Growth Factor Eye Drops in Patients with Stage 2 or 3 Neurotrophic Keratitis. Presented at the Congress of the European Society of Ophthalmology (SOE), Barcelona, Spain, June, 10–13, 2017.
4 U.S. Prescribing Information
5 McKay TB, Seyed-Razavi Y, Ghezzi CE, Dieckmann G, Nieland TJF, Cairns DM, Pollard RE, Hamrah P, Kaplan DL. Corneal pain and experimental model development. Prog Retin Eye Res. 2018 Nov 16. pii: S1350-9462(18)30013-2. [Epub ahead of print]
6 Mastropasqua L, Massaro-Giordano G, Nubile M, Sacchetti M. Understanding the Pathogenesis of Neurotrophic Keratitis: The Role of Corneal Nerves. Journal of Cellular Physiology. 2017;232(4):717-24.
US-NK20190103