US FDA APPROVES ASTRAZENECA’S CALQUENCE® (acalabrutinib) FOR ADULT PATIENTS WITH PREVIOUSLY-TREATED MANTLE CELL LYMPHOMA
Accelerated approval of Bruton tyrosine kinase (BTK) inhibitor in MCL marks AstraZeneca’s entry into the treatment of blood cancers
80% of patients receiving CALQUENCE achieved an overall response, with 40% achieving a complete response
(WILMINGTON, Del., October 31, 2017) – AstraZeneca and its hematology research and development center of excellence, Acerta Pharma, today announced that the US Food and Drug Administration (FDA) has granted accelerated approval to CALQUENCE® (acalabrutinib). CALQUENCE is a kinase inhibitor indicated for the treatment of adult patients with mantle cell lymphoma (MCL) who have received at least one prior therapy.1
CALQUENCE is approved under the FDA’s accelerated approval pathway, based on overall response rate, which allows for earlier approval of medicines that treat serious conditions and that fill an unmet medical need based on a surrogate endpoint.2 Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.1
Pascal Soriot, Chief Executive Officer of AstraZeneca, said: “The accelerated approval of CALQUENCE is a landmark moment for our company. It provides an exciting new treatment option for patients with mantle cell lymphoma and marks the first approval of a medicine that will be the cornerstone of our presence in hematology. Furthermore, today’s approval demonstrates our commitment to scientific leadership in Oncology and reinforces our progress towards returning to growth.”
Michael L. Wang, MD, Professor, Department of Lymphoma/Myeloma, The University of Texas MD Anderson Cancer Center, and Principal Investigator of the ACE-LY-004 MCL clinical trial, said: “The acalabrutinib approval represents an important development for patients currently battling mantle cell lymphoma, an aggressive type of blood cancer that is typically diagnosed at an advanced stage and associated with a high relapse rate. In addition to the overall response rate, the high complete response rate of 40% seen in this trial illustrates the potential of acalabrutinib to help patients.”
Summary of key efficacy results as assessed by Independent Review Committee (IRC) from the ACE-LY-004 trial,1 a Phase II open-label, single-arm clinical trial in 124 adult patients with relapsed or refractory MCL:
| Efficacy Measure | IRC Results |
| Overall Response Rate | 80% (95% CI: 72, 87) |
| Complete Response | 40% (95% CI: 31, 49) |
| Partial Response | 40% (95% CI: 32, 50) |
Per 2014 Lugano Classification, CI = Confidence Interval
In the ACE-LY-004 trial, the most common adverse reactions (≥20%) of any grade were anemia (46%), thrombocytopenia (44%), headache (39%), neutropenia (36%), diarrhea (31%), fatigue (28%), myalgia (21%), and bruising (21%).1 Hematological events were based on laboratory measurements and adverse reactions.1
Dosage reductions or discontinuations due to any adverse reaction were reported in 1.6% and 6.5% of patients, respectively.1 Increases in creatinine 1.5 to 3 times the upper limit of normal occurred in 4.8% of patients.1
These data demonstrate the potential impact that CALQUENCE could have on the management of previously-treated MCL.
Meghan Gutierrez, Chief Executive Officer, Lymphoma Research Foundation, said: “Relapse is common in mantle cell lymphoma patients and represents disease progression.3 When patients learn there is a new treatment option available for their disease, it brings great hope and an opportunity to participate in shared decision making with their healthcare team.”
Full results from the ACE-LY-004 clinical trial have been submitted for presentation at a forthcoming medical meeting. This will be the first MCL trial data to be presented from the acalabrutinib development program, which includes both monotherapy and combination therapies in a broad range of blood cancers and solid tumors. Acalabrutinib is also being evaluated in combination with bendamustine and rituximab as a potential 1st-line treatment for patients with MCL in the Phase III ACE-LY-308 clinical trial.4
IMPORTANT SAFETY INFORMATION ABOUT CALQUENCE
Hemorrhage
Serious hemorrhagic events, including fatal events, have occurred in the combined safety database of 612 patients with hematologic malignancies treated with CALQUENCE monotherapy. Grade 3 or higher bleeding events, including gastrointestinal, intracranial, and epistaxis, have been reported in 2% of patients. Overall, bleeding events, including bruising and petechiae of any grade, occurred in approximately 50% of patients with hematological malignancies.
The mechanism for the bleeding events is not well understood.
CALQUENCE may further increase the risk of hemorrhage in patients receiving antiplatelet or anticoagulant therapies, and patients should be monitored for signs of bleeding.
Consider the benefit-risk of withholding CALQUENCE for 3 to 7 days pre- and post-surgery, depending upon the type of surgery and the risk of bleeding.
Infection
Serious infections (bacterial, viral, or fungal), including fatal events and opportunistic infections, have occurred in the combined safety database of 612 patients with hematologic malignancies treated with CALQUENCE monotherapy. Grade 3 or higher infections occurred in 18% of these patients. The most frequently reported Grade 3 or 4 infection was pneumonia. Infections due to hepatitis B virus (HBV) reactivation and progressive multifocal leukoencephalopathy (PML) have occurred.
Monitor patients for signs and symptoms of infection and treat as medically appropriate. Consider prophylaxis in patients who are at increased risk for opportunistic infections.
Cytopenias
In the combined safety database of 612 patients with hematologic malignancies, patients treated with CALQUENCE monotherapy experienced Grade 3 or 4 cytopenias, including neutropenia (23%), anemia (11%), and thrombocytopenia (8%), based on laboratory measurements. Monitor complete blood counts monthly during treatment.
Second Primary Malignancies
Second primary malignancies, including non-skin carcinomas, have occurred in 11% of patients with hematologic malignancies treated with CALQUENCE monotherapy in the combined safety database of 612 patients. The most frequent second primary malignancy was skin cancer, reported in 7% of patients. Advise protection from sun exposure.
Atrial Fibrillation and Flutter
In the combined safety database of 612 patients with hematologic malignancies treated with CALQUENCE monotherapy, atrial fibrillation and atrial flutter of any grade occurred in 3% of patients, and Grade 3 in 1% of patients. Monitor for atrial fibrillation and atrial flutter and manage as appropriate.
ADVERSE REACTIONS
The most common adverse reactions (≥20%) of any grade were anemia,* thrombocytopenia,* headache (39%), neutropenia,* diarrhea (31%), fatigue (28%), myalgia (21%), and bruising (21%).
*Treatment-emergent decreases (all grades) of hemoglobin (46%), platelets (44%), and neutrophils (36%) were based on laboratory measurements and adverse reactions.
The most common Grade ≥ 3 non-hematological adverse reaction (reported in at least 2% of patients) was diarrhea (3.2%).
Dosage reductions or discontinuations due to any adverse reaction were reported in 1.6% and 6.5% of patients, respectively.
Increases in creatinine 1.5 to 3 times the upper limit of normal occurred in 4.8% of patients.
DRUG INTERACTIONS
Strong CYP3A Inhibitors: Avoid co-administration with a strong CYP3A inhibitor. If a strong CYP3A inhibitor will be used short-term, interrupt CALQUENCE.
Moderate CYP3A Inhibitors: When CALQUENCE is co-administered with a moderate CYP3A inhibitor, reduce CALQUENCE dose to 100 mg once daily.
Strong CYP3A Inducers: Avoid co-administration with a strong CYP3A inducer. If a strong CYP3A inducer cannot be avoided, increase the CALQUENCE dose to 200 mg twice daily.
Gastric Acid Reducing Agents: If treatment with a gastric acid reducing agent is required, consider using an H2-receptor antagonist or an antacid. Take CALQUENCE 2 hours before taking an H2-receptor antagonist. Separate dosing with an antacid by at least 2 hours.
Avoid co-administration with proton pump inhibitors. Due to the long-lasting effect of proton pump inhibitors, separation of doses may not eliminate the interaction with CALQUENCE.
SPECIFIC POPULATIONS
There is insufficient clinical data on CALQUENCE use in pregnant women to inform a drug-associated risk for major birth defects and miscarriage. Advise women of the potential risk to a fetus.
It is not known if CALQUENCE is present in human milk. Advise lactating women not to breastfeed while taking CALQUENCE and for at least 2 weeks after the final dose.
Please see complete Prescribing Information including Patient Information.
– ENDS –
NOTES TO EDITORS
About CALQUENCE®
(acalabrutinib)
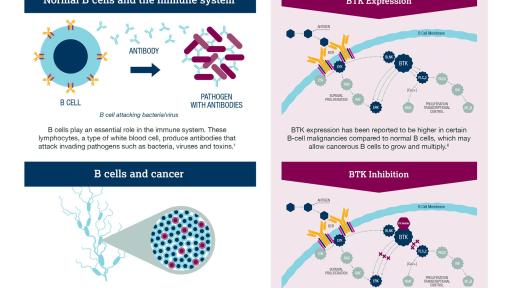
CALQUENCE (acalabrutinib, previously known as ACP-196) is an inhibitor of Bruton tyrosine kinase (BTK). CALQUENCE binds covalently to BTK, thereby inhibiting its activity.1 In B cells, BTK signaling results in activation of pathways necessary for B cell proliferation, trafficking, chemotaxis, and adhesion.1
The recommended dose of CALQUENCE is one 100mg capsule taken orally approximately every twelve hours until disease progression or unacceptable toxicity.1 CALQUENCE may be taken with or without food.1
Acalabrutinib is also in development for the treatment of multiple B-cell malignancies and other cancers including chronic lymphocytic leukemia, MCL, Waldenström macroglobulinemia, follicular lymphoma, diffuse large B-cell lymphoma, and multiple myeloma. It is also being studied as a monotherapy and in combination trials for the treatment of solid tumors. More than 35 clinical trials across 40 countries with more than 2,500 patients are underway or have completed.5
CALQUENCE was granted Orphan Drug Designation for the treatment of adult patients with MCL in September 2015 and Breakthrough Therapy Designation in August 2017 by the US FDA for the treatment of adult patients with MCL who have received at least one prior therapy.
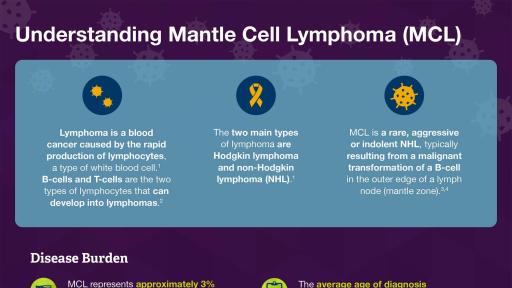
About Mantle Cell Lymphoma (MCL)
MCL is an aggressive B-cell non-Hodgkin lymphoma (NHL) with poor prognosis.6,7,8,9 MCL accounts for approximately 3% of new NHL cases in the US, with approximately 3,300 new cases diagnosed each year.10,11 The median age at diagnosis is 68 years, with a 3:1 male predominance.7 While MCL patients initially respond to treatment, there is a high relapse rate.7
About the ACE-LY-004 Trial
ACE-LY-004 is a Phase II open-label, single-arm clinical trial in 124 adult patients with relapsed or refractory MCL. The trial showed that 80% (95% CI: 72, 87) of patients treated with CALQUENCE achieved an overall response; 40% (95% CI: 31, 49) achieved a complete response and 40% (95% CI: 32, 50) achieved a partial response, per 2014 Lugano classification as assessed by Independent Review Committee.1
About Acerta Pharma
Acerta Pharma, a member of the AstraZeneca Group, is creating novel therapies intended for the treatment of cancer and autoimmune diseases. AstraZeneca acquired a majority stake interest in Acerta Pharma, which serves as AstraZeneca’s hematology research and development center of excellence. For more information, please visit www.acerta-pharma.com.
About AstraZeneca in Oncology
AstraZeneca has a deep-rooted heritage in Oncology and offers a quickly growing portfolio of new medicines that have the potential to transform patients’ lives and the Company’s future. With at least six new medicines to be launched between 2014 and 2020 and a broad pipeline of small molecules and biologics in development, we are committed to advance New Oncology as one of AstraZeneca’s five Growth Platforms focused on lung, ovarian, breast and blood cancers. In addition to our core capabilities, we actively pursue innovative partnerships and investments that accelerate the delivery of our strategy as illustrated by our investment in Acerta Pharma in hematology.
By harnessing the power of four scientific platforms – Immuno-Oncology, Tumor Drivers and Resistance, DNA Damage Response and Antibody Drug Conjugates – and by championing the development of personalized combinations, AstraZeneca has the vision to redefine cancer treatment and one day eliminate cancer as a cause of death.
About AstraZeneca
AstraZeneca is a global, science-led biopharmaceutical company that focuses on the discovery, development and commercialization of prescription medicines, primarily for the treatment of diseases in three main therapy areas – Oncology, Cardiovascular & Metabolic Diseases and Respiratory. The Company also is selectively active in the areas of autoimmunity, neuroscience and infection. AstraZeneca operates in over 100 countries and its innovative medicines are used by millions of patients worldwide.
For more information, please visit www.astrazeneca-us.com and follow us on Twitter @AstraZenecaUS.
Media Inquiries
Michele Meixell
+1 302 885 2677
- CALQUENCE® (acalabrutinib) Prescribing Information. AstraZeneca Pharmaceuticals LP, Wilmington, DE.
- US Food and Drug Administration. Guidance for Industry Labeling for Human Prescription Drug and Biological Products Approved Under the Accelerated Approval Regulatory pathway. Available online. Accessed August 2017.
- Lymphoma Research Foundation. Getting the Facts Mantle Cell Lymphoma: Relapsed/Refractory. Available online. Accessed July 2017.
- Acerta Pharma BV. A Study of Bendamustine and Rituximab Alone Versus in Combination With Acalabrutinib in Subjects With Previously Untreated Mantle Cell Lymphoma. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available online. Accessed June 2017.
- Data on File. REF US-15441. AstraZeneca Pharmaceuticals LP, Wilmington, DE.
- Leukemia & Lymphoma Society. Mantle Cell Lymphoma Facts. Available online. Accessed June 2017.
- Cheah CY, Seymour JF, Wang M. Mantle Cell Lymphoma. Journal of Clinical Oncology 34, no. 11 (April 2016) 1256-1269.
- Hoster E, Klapper W et al. Confirmation of the Mantle-Cell Lymphoma International Prognostic Index in Randomized Trials of the European Mantle-Cell Lymphoma Network. Journal of Clinical Oncology 2014;32:1338-1346.
- Dreyling M, Ferrero S. The role of targeted treatment in mantle cell lymphoma: is transplant dead or alive? Haematologica 2016 Volume 101(2):104-114.
- Zhou Y, Wang H, Fang W, et al. Incidence trends of mantle cell lymphoma in the United States between 1992 and 2004. Cancer. 2008;113(4):791-798.
- Teras LR, DeSantis CE, Cerhan JR, et al. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66:443-459.
US-12367 Last Updated 10/17