TESARO ANNOUNCES U.S. FDA APPROVAL OF ZEJULA™ (NIRAPARIB) FOR WOMEN WITH RECURRENT OVARIAN CANCER
-
ZEJULA is the first and only PARP inhibitor to be approved for the maintenance treatment of women with recurrent ovarian cancer
-
Use of ZEJULA does not require patient selection with a biomarker test
-
U.S. commercial launch planned for late April
-
Investor conference call and webcast to be held at 4:30 PM ET today
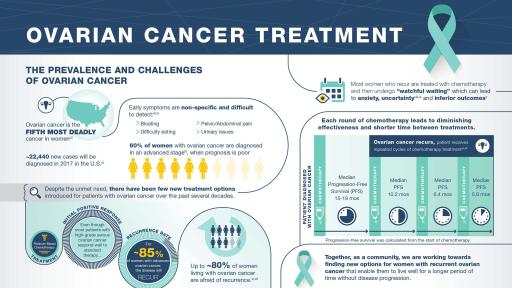
WALTHAM, MA, March 27, 2017 – TESARO, Inc. (NASDAQ: TSRO), an oncology-focused biopharmaceutical company, today announced that the U.S. Food and Drug Administration (FDA) has approved ZEJULA™ (niraparib), an oral, once-daily poly(ADP-ribose) polymerase (PARP) inhibitor, for the maintenance treatment of women with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in a complete or partial response (CR or PR) to platinum-based chemotherapy. ZEJULA is the first PARP inhibitor to be approved by the FDA that does not require BRCA mutation or other biomarker testing. TESARO anticipates launching ZEJULA in the United States in late April.
Multimedia materials accompanying this release are available at: https://www.multivu.com/players/English/8050551-tesaro-zejula-niraparib-fda-approval/.
ZEJULA is the only PARP inhibitor that has demonstrated a clinically meaningful increase in progression-free survival (PFS) in women with recurrent ovarian cancer, regardless of BRCA mutation or biomarker status, in a randomized, prospectively designed Phase 3 clinical trial. ZEJULA offers oral, once-daily dosing, enabling convenient administration for maintenance treatment. FDA approval of ZEJULA is based upon data from the international Phase 3 ENGOT-OV16/NOVA trial, a double-blind, placebo-controlled study that enrolled 553 patients with recurrent ovarian cancer who had achieved either a PR or CR to their most recent platinum-based chemotherapy. Approximately two-thirds of study participants did not have germline BRCA mutations. Progression in the NOVA study was determined by robust, unbiased, blinded central review, to be the earlier of radiographic or clinical progression. ZEJULA significantly increased PFS in patients with and without germline BRCA mutations as compared to control. Treatment with ZEJULA reduced the risk of disease progression or death by 74% in patients with germline BRCA mutations (HR 0.26) and by 55% in patients without germline BRCA mutations (HR 0.45). The magnitude of benefit was similar for patients entering the trial with a PR or a CR.
The most common grade 3/4 adverse reactions to ZEJULA in the NOVA trial included thrombocytopenia (29%), anemia (25%), neutropenia (20%), and hypertension (9%). The majority of hematologic adverse events were successfully managed via dose modification, and discontinuation of therapy due to thrombocytopenia, neutropenia and anemia occurred in 3.3%, 1.9% and 1.4% of patients, respectively. Following dose adjustment based on individual tolerability, the incidence of grade 3/4 thrombocytopenia was low; approximately <1% after month 2. Please see the “Select Important Safety Information” below for warnings, precautions and adverse events.
“We are so gratified to bring this unique new medicine to women with ovarian cancer, and would like to thank the patients who gave selflessly to participate in this trial with the assistance of their caregivers and physicians. We consider clinical trial participants to be the most important contributors to the success of the ZEJULA clinical development program,” said Mary Lynne Hedley, Ph.D., President and Chief Operating Officer of TESARO. “We would also like to express our appreciation to the FDA for its rapid and thorough assessment of the ZEJULA application in less than three months after it was accepted for review, as well as our partners at ENGOT for their diligence and care in executing the NOVA clinical trial. TESARO is committed to supporting women bravely facing ovarian cancer and we are planning to work with patients, healthcare providers and payers to ensure access to this paradigm-changing medicine.”
“Despite high response rates to platinum-based treatment in recurrent ovarian cancer patients, the effectiveness of such chemotherapy diminishes over time. Unfortunately, progression free survival generally gets shorter after each subsequent treatment with a platinum-based chemotherapy regimen. Therefore, a treatment like ZEJULA that can increase progression-free survival after platinum therapy is very meaningful to patients and their families,” said Dr. Ursula Matulonis, M.D., Director, Gynecologic Oncology at Dana-Farber Cancer Institute and Professor of Medicine, Harvard Medical School. “Until recently, there have been few treatment advances for women with recurrent ovarian cancer and even fewer options available for women who do not harbor BRCA mutations. We are excited to have the opportunity to offer appropriate patients an oral, once-daily maintenance treatment that reduces the risk of cancer progression and extends the time between courses of chemotherapy for patients who have few treatment options.”
“The approval of ZEJULA, the first maintenance therapy approved in the U.S. for recurrent ovarian cancer, is extremely encouraging for the ovarian cancer community,” said Dr. Mansoor Raza Mirza, M.D., ENGOT-OV16/NOVA Study Chair and Medical Director of the Nordic Society of Gynaecological Oncology (NSGO). “The unique design of the NOVA study, which included women both with and without germline BRCA mutations, allowed us to determine that ZEJULA provides significant progression-free survival improvement in a very broad patient population. Having the option of prescribing ZEJULA without the need for a diagnostic test could fundamentally change the way we treat this disease from ‘watch and wait’ after a response to chemotherapy, to active treatment. With the significant increase in PFS observed in NOVA, I believe that we are changing the course of disease for patients with ovarian cancer, regardless of platinum sensitivity and independent of BRCA mutation or biomarker status.”
“Women with recurrent ovarian cancer often experience considerable fear and anxiety about future recurrences,” said Audra Moran, President and CEO of Ovarian Cancer Research Fund Alliance. “ZEJULA may offer patients and their families a treatment option during this stressful period. The ovarian cancer community is eager for new treatment options, and we are glad that ZEJULA will be available to women that have completed their platinum-based chemotherapy.”
The full prescribing information for ZEJULA will be made available at www.zejula.com. ZEJULA is administered at a starting dose of 300 milligrams once per day, which can be modified based upon individual tolerability. TESARO anticipates that ZEJULA will be made available in the U.S. in late April. A Marketing Authorisation Application for niraparib is under review by the European Medicines Agency (EMA).
TESARO Investor Conference Call and Webcast
TESARO will webcast a conference call with investors and analysts today, March 27, 2017 at 4:30 PM ET. Investors and analysts may access this call by dialing (877) 853-5334 (U.S. and Canada) or (970) 315-0307 (international); no passcode is necessary. During this conference call, TESARO management will review the approval of ZEJULA and answer questions from investors and analysts. This event will be webcast live and archived for 30 days, and may be accessed from the TESARO Investor Events and Presentations webpage at www.tesarobio.com.
About Ovarian Cancer
Approximately 22,000 women are diagnosed with ovarian cancer each year in the United States, and more than 65,000 women are diagnosed annually in Europe. Ovarian cancer is the fifth-most frequent cause of cancer death among women. Despite high response rates to platinum-based chemotherapy in the second-line advanced treatment setting, approximately 85% of patients will experience recurrence within two years. Per NCCN guidelines, BRCA testing and genetic counseling remain important components of the medical workup for all patients upon a diagnosis with ovarian cancer.
About the ZEJULA™ (Niraparib) ENGOT-OV16/NOVA Clinical Trial
ENGOT-OV16/NOVA was a double-blind, placebo-controlled, international Phase 3 trial of niraparib that enrolled 553 patients with recurrent ovarian cancer who were in a response to their most recent platinum-based chemotherapy. Patients were enrolled into one of two independent cohorts based on germline BRCA mutation status. One cohort enrolled patients who were germline BRCA mutation carriers (gBRCAmut), and the second cohort enrolled patients who were not germline BRCA mutation carriers (non-gBRCAmut) and included patients with HRD-positive and HRD-negative tumors. Within each cohort, patients were randomized 2:1 to receive niraparib or placebo and were treated continuously with placebo or 300 milligrams of niraparib, dosed as three 100 milligram tablets once per day, until progression. The primary endpoint of this study was progression-free survival (PFS). Secondary endpoints included patient-reported outcomes, chemotherapy-free interval length, PFS 2, overall survival, and other measures of safety and tolerability. More information about this trial is available at http://clinicaltrials.gov/show/NCT01847274.
Among patients who were germline BRCA mutation carriers, the niraparib arm successfully achieved statistical significance over the control arm for the primary endpoint of PFS, with a hazard ratio of 0.26 (95% CI, 0.173-0.410). The median PFS for patients treated with niraparib was 21.0 months, compared to 5.5 months for control (p<0.0001). Niraparib also showed statistical significance for patients in the non-germline BRCA mutant cohort. The niraparib arm successfully achieved statistical significance over the control arm for the primary endpoint of PFS, with a hazard ratio of 0.45 (95% CI, 0.338-0.607). The median PFS for patients treated with niraparib was 9.3 months, compared to 3.9 months for control (p<0.0001). Secondary endpoint analyses, including chemotherapy-free interval, time to first subsequent treatment, and PFS 2 were all statistically significant and favored niraparib over control for patients in both the gBRCAmut and non-gBRCAmut cohorts. Patient-reported outcome results from validated survey tools indicated that niraparib-treated patients reported no difference from control in measures associated with quality of life.
The full results of the ENGOT-OV16/NOVA trial were presented in detail at the European Society for Medical Oncology (ESMO) 2016 Congress in Copenhagen on October 8, 2016 by Dr. Mansoor Raza Mirza, M.D., Medical Director of the Nordic Society of Gynecologic Oncology (NSGO) and principal investigator. These data were simultaneously published in the New England Journal of Medicine.
Select Important Safety Information
Myelodysplastic Syndrome/Acute Myeloid Leukemia (MDS/AML) was reported in patients treated with ZEJULA in all clinical studies. Discontinue ZEJULA if MDS/AML is confirmed.
Hematologic adverse reactions (thrombocytopenia, anemia and neutropenia) have been reported in patients treated with ZEJULA. Do not start ZEJULA until patients have recovered from hematological toxicity caused by previous chemotherapy (≤ Grade 1). Monitor complete blood counts weekly for the first month, monthly for the next 11 months of treatment, and periodically after this time.
Hypertension and hypertensive crisis have been reported in patients treated with ZEJULA. Monitor blood pressure and heart rate monthly for the first year and periodically thereafter during treatment with ZEJULA. Closely monitor patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension.
Based on its mechanism of action, ZEJULA can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception during treatment and for six months after receiving the final dose. Because of the potential for serious adverse reactions in breastfed infants from ZEJULA, advise a lactating woman not to breastfeed during treatment with ZEJULA and for one month after receiving the final dose.
In clinical studies, the most common adverse reactions included: thrombocytopenia, anemia, neutropenia, nausea, constipation, vomiting, abdominal pain/distension, mucositis/stomatitis, diarrhea, fatigue/asthenia, decreased appetite, headache, insomnia, nasopharyngitis, dyspnea, rash and hypertension.
Please see full Prescribing Information for additional Safety Information.
Additional Clinical Trials of ZEJULA (Niraparib)
TESARO is building a robust niraparib franchise by assessing activity across multiple tumor types and by evaluating several potential combinations of niraparib with other therapeutics. The ongoing development program for niraparib includes a Phase 3 trial in patients who have received first-line treatment for ovarian cancer (the PRIMA trial) and a registrational Phase 2 trial in patients who have received multiple lines of treatment for ovarian cancer (the QUADRA trial). Several combination studies are also underway, including trials of niraparib plus pembrolizumab (the TOPACIO trial) and niraparib plus bevacizumab (the AVANOVA trial). Janssen Biotech has licensed rights to develop and commercialize niraparib specifically for patients with prostate cancer worldwide, except in Japan.
About ZEJULA (Niraparib)
ZEJULA is an oral, once-daily poly(ADP-ribose) polymerase (PARP) 1/2 inhibitor that is indicated in the U.S. for the maintenance treatment of adult patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in a complete or partial response to platinum-based chemotherapy. In preclinical studies, ZEJULA concentrates in the tumor relative to plasma, delivering greater than 90% durable inhibition of PARP 1/2 and a persistent antitumor effect.
TOGETHER with TESARO™
TOGETHER with TESARO™ is a patient resource program dedicated to supporting people living with cancer. The program assists with access issues, so that patients with cancer can be free to focus on treatment goals and simply living life. It provides a full suite of services to meet each patient’s needs and individual experience. A team of access and affordability experts is available to help oncology practices and patients gain access to the medication they require. TOGETHER with TESARO will continue to evolve and grow to meet provider and patient needs. For more information, please visit www.togetherwithtesaro.com or call 1-844-2TESARO (1-844-283-7276).
About TESARO
TESARO is an oncology-focused biopharmaceutical company devoted to providing transformative therapies to people bravely facing cancer. For more information, visit www.tesarobio.com, and follow us on Twitter and LinkedIn.
Investor/Media Contact:
Jennifer Davis
Vice President, Corporate Affairs & Investor Relations
+1.781.325.1116 or [email protected]
Forward Looking Statements
To the extent that statements contained in this press release are not descriptions of historical facts regarding TESARO, they are forward-looking statements reflecting the current beliefs and expectations of management made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Words such as "may," "will," "expect," "anticipate," "estimate," "intend," and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward-looking statements. Forward-looking statements in this release involve substantial risks and uncertainties that could cause our clinical development programs, future results, performance or achievements to differ significantly from those expressed or implied by the forward-looking statements. Such risks and uncertainties include, among others, risks associated with competition in the PARP market, risks related to pricing and reimbursement, risks related to manufacturing and supply, risks related to intellectual property, and other risks and uncertainties that could affect the availability or commercial potential of ZEJULA. TESARO undertakes no obligation to update or revise any forward-looking statements. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to the business of the Company in general, see TESARO's Annual Report on Form 10-K for the year ended December 31, 2016.