Shire’s Investor Day Showcases Strength of Rare Disease Pipeline and Commercial Portfolio
Company spotlights serial innovation in rare diseases, features Hematology and Immunology franchises
PR Newswire, Lexington, Mass. – November 10, 2016
Shire plc (LSE: SHP, NASDAQ: SHPG) today will host an Investor Day focused on its commercial portfolio and innovative R&D pipeline, including highlights from its late-stage clinical portfolio, which together support its long term growth aspirations.
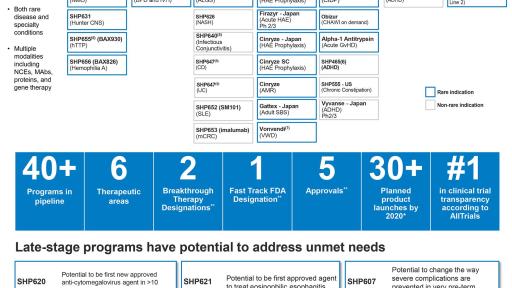
“Over the last three years, Shire’s sharp focus has helped us build an industry leading rare disease pipeline,” said Flemming Ornskov, M.D., M.P.H., Chief Executive Officer. “Our pipeline, which is the most robust in Shire’s history, includes more than 40 clinical programs, over 50% of which are in registration or Phase 3, and 70% of which are in rare diseases. We focus on delivering first- and best- in-class medicines to address significant unmet need for patients around the world to achieve maximum impact.”
“Our deep understanding of disease and our serial innovation in strategic areas of focus have allowed us to advance molecules in some of the most difficult-to-treat and life-threatening conditions,” said Philip J. Vickers, Ph.D., Global Head of Research & Development. “Our internal innovation engine, with the help of our external partners, is driving forward a robust clinical development portfolio and enabling us to bring our medicines to patients who need them.”
Franchise Highlights
Hematology
Shire has deep expertise and established leadership in Hematology with an industry-leading portfolio across segments including Hemophilia A, inhibitors, Hemophilia B and von Willebrand disease. In Hemophilia A, Shire is focused on addressing unmet needs while raising the standards of care through increased diagnosis, prophylaxis and personalization of treatment with ADVATE [Antihemophilic Factor (Recombinant)] and ADYNOVATE [Antihemophilic Factor (Recombinant), PEGylated]. In Hemophilia with inhibitors, FEIBA [Anti-Inhibitor Coagulant Complex] offers a valued bypassing therapy with a unique mechanism and is the only bypass widely approved for both prophylaxis and on-demand treatment. The Company’s Hematology products are available today in more than 100 countries around the world, and launches (both product and new indication) are planned in more than 40 countries by 2018. Shire’s future innovation in rare hematology includes non-factor based therapies and gene therapy.
Immunology
In Primary Immunodeficiency (PI), Shire has one of the broadest portfolios approved for self-infusion, including CUVITRU [Immune Globulin Subcutaneous (Human)], 20% Solution; GAMMAGARD LIQUID [Immune Globulin Infusion (Human)] 10%; SUBCUVIA; and HYQVIA [Immune Globulin Infusion 10% (Human) with Recombinant Human Hyaluronidase]. With a growing need for more treatment options and a shift from hospital to home care, Shire is focused on improving diagnoses, providing individualized treatment and supporting patients to help further expand its leadership position in Immunology.
Late-Stage Clinical Portfolio
SHP621
Shire is conducting a Phase 3 induction study (SHP621-301) in Eosinophilic Esophagitis (EoE) and simultaneously enrolling a Phase 3 treatment extension study (SHP621-302). EoE is a chronic, immune/antigen-mediated esophageal disease characterized clinically by symptoms related to esophageal dysfunction. The Company has already completed Phase 2 trials that met co-primary endpoints as well as an open-label extension study. SHP621 was granted Breakthrough Therapy Designation by the FDA in May 2016 for the treatment of EoE.
SHP647
SHP647 is a novel biologic for the treatment of inflammatory bowel disease (IBD) and the only anti-integrin directly targeting MAdCAM-1 with a potentially differentiated and improved safety profile compared to current treatments. Shire plans to begin Phase 3 development of SHP647 in 2017.
SHP620 (maribavir)
Shire is planning two Phase 3 studies with maribavir for the treatment of cytomegalovirus (CMV) infection in transplant recipient patients. CMV infection can cause serious illness in transplant recipient patients and other patients with compromised immune systems. Use of currently available anti-CMV drugs is limited due to toxicity, particularly in those patients with resistant or refractory disease. All clinical studies of maribavir have shown an acceptable safety profile and potent antiviral activity including against strains of CMV resistant or refractory to other anti-CMV agents.
SHP607
Shire intends to continue to pursue the development of SHP607 to potentially prevent certain severe complications in very pre-term infants. The Company reported topline results from its Phase 2 clinical study in June 2016. The study did not meet its primary endpoint, reduction in severity of retinopathy of prematurity; however, it demonstrated clinically relevant effects on secondary endpoints, including development of severe bronchopulmonary dysplasia (BPD) and intraventricular hemorrhage (IVH).
SHP465
Shire plans to resubmit SHP465 for FDA approval in Q4 2016, with a projected launch in the second half of 2017. SHP465 is a novel three-bead formulation which aims to provide a long-acting therapeutic option for the treatment of attention-deficit/hyperactivity disorder (ADHD). The Company has conducted two Phase 3 studies and one open label Phase 1 study to support resubmission, all of which successfully met their primary and key secondary endpoints.
SHP643
Shire is conducting a single pivotal trial of SHP643, formerly DX-2930, together with an open-label extension study for prophylaxis of hereditary angioedema (HAE) with the aim of FDA approval in 2018, subject to clinical trial results. HAE is a rare, debilitating genetic inflammatory condition which causes episodes of swelling in the face, extremities, and GI tract and can be life threatening. The Company has demonstrated proof of concept for SHP643 in long-term prophylaxis of HAE without any significant safety concerns to date. SHP643 has received both Orphan Drug Designation and Breakthrough Therapy Designation from the FDA.
Meeting Webcast
These presentations will be broadcast via a live video and audio webcast that can be accessed under the “Presentations & Webcasts” tab in the Investor Relations section of the Company’s website at www.shire.com. A replay of the webcast will be archived on the website following the presentation.
The details of the call are as follows:
| UK dial in: | 0808 237 0036 or 020 3426 2889 |
| US dial in: | 1 877 841 4559 or 1 347 329 1282 |
| Live video and audio webcast: | shireinvestorday.com |
For further information please contact:
| Investor Relations |
|
|
|
Sarah Elton-Farr |
+44 1256 894157 |
|
|
Ian Karp |
+1 781 482 9018 |
|
|
Robert Coates |
+44 1256 894874 |
|
|
Media |
|
|
|
Lisa Adler |
+1 617 588 8607 |
|
|
Debbi Ford |
+1 617 949 9083 |
NOTES TO EDITORS
Shire is the leading global biotechnology company focused on serving people with rare diseases and other highly specialized conditions. We strive to develop best-in-class products, many of which are available in more than 100 countries, across core therapeutic areas including Hematology, Immunology, Neuroscience, Ophthalmics, Lysosomal Storage Disorders, Gastrointestinal / Internal Medicine / Endocrine and Hereditary Angioedema; and a growing franchise in Oncology.
Our employees come to work every day with a shared mission: to develop and deliver breakthrough therapies for the hundreds of millions of people in the world affected by rare diseases and other high-need conditions, and who lack effective therapies to live their lives to the fullest.
Forward-Looking Statements
Statements included herein that are not historical facts, including without limitation statements concerning future strategy, plans, objectives, expectations and intentions, the anticipated timing of clinical trials and approvals for, and the commercial potential of, inline or pipeline products are forward-looking statements. Such forward-looking statements involve a number of risks and uncertainties and are subject to change at any time. In the event such risks or uncertainties materialize, Shire’s results could be materially adversely affected. The risks and uncertainties include, but are not limited to, the following:
- Shire’s products may not be a commercial success;
- increased pricing pressures and limits on patient access as a result of governmental regulations and market developments may affect Shire’s future revenues, financial condition and results of operations;
- Shire conducts its own manufacturing operations for certain of its products and is reliant on third party contract manufacturers to manufacture other products and to provide goods and services. Some of Shire’s products or ingredients are only available from a single approved source for manufacture. Any disruption to the supply chain for any of Shire’s products may result in Shire being unable to continue marketing or developing a product or may result in Shire being unable to do so on a commercially viable basis for some period of time;
- the manufacture of Shire’s products is subject to extensive oversight by various regulatory agencies. Regulatory approvals or interventions associated with changes to manufacturing sites, ingredients or manufacturing processes could lead to significant delays, an increase in operating costs, lost product sales, an interruption of research activities or the delay of new product launches;
- certain of Shire’s therapies involve lengthy and complex processes, which may prevent Shire from timely responding to market forces and effectively managing its production capacity;
- Shire has a portfolio of products in various stages of research and development. The successful development of these products is highly uncertain and requires significant expenditures and time, and there is no guarantee that these products will receive regulatory approval;
- the actions of certain customers could affect Shire’s ability to sell or market products profitably. Fluctuations in buying or distribution patterns by such customers can adversely affect Shire’s revenues, financial conditions or results of operations;
- Shire’s products and product candidates face substantial competition in the product markets in which it operates, including competition from generics;
- adverse outcomes in legal matters, tax audits and other disputes, including Shire’s ability to enforce and defend patents and other intellectual property rights required for its business, could have a material adverse effect on the combined company’s revenues, financial condition or results of operations;
- inability to successfully compete for highly qualified personnel from other companies and organizations;
- failure to achieve the strategic objectives with respect to Shire’s acquisition of NPS Pharmaceuticals, Inc., Dyax Corp. (“Dyax”) or Baxalta Inc. (“Baxalta”)may adversely affect Shire’s financial condition and results of operations;
- Shire’s growth strategy depends in part upon its ability to expand its product portfolio through external collaborations, which, if unsuccessful, may adversely affect the development and sale of its products;
- a slowdown of global economic growth, or economic instability of countries in which Shire does business, as well as changes in foreign currency exchange rates and interest rates, that adversely impact the availability and cost of credit and customer purchasing and payment patterns, including the collectability of customer accounts receivable;
- failure of a marketed product to work effectively or if such a product is the cause of adverse side effects could result in damage to the Shire’s reputation, the withdrawal of the product and legal action against Shire;
- investigations or enforcement action by regulatory authorities or law enforcement agencies relating to Shire’s activities in the highly regulated markets in which it operates may result in significant legal costs and the payment of substantial compensation or fines;
- Shire is dependent on information technology and its systems and infrastructure face certain risks, including from service disruptions, the loss of sensitive or confidential information, cyber-attacks and other security breaches or data leakages that could have a material adverse effect on Shire’s revenues, financial condition or results of operations;
- Shire incurred substantial additional indebtedness to finance the Baxalta acquisition, which may decrease its business flexibility and increase borrowing costs;
- difficulties in integrating Dyax or Baxalta into Shire may lead to the combined company not being able to realize the expected operating efficiencies, cost savings, revenue enhancements, synergies or other benefits at the time anticipated or at all; and
other risks and uncertainties detailed from time to time in Shire’s filings with the Securities and Exchange Commission, including those risks outlined in “ITEM 1A: Risk Factors” in Shire’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2016.
All forward-looking statements attributable to us or any person acting on our behalf are expressly qualified in their entirety by this cautionary statement. Readers are cautioned not to place undue reliance on these forward-looking statements that speak only as of the date hereof. Except to the extent otherwise required by applicable law, we do not undertake any obligation to update or revise forward-looking statements, whether as a result of new information, future events or otherwise.