| Media Relations: | Investor Relations: |
|---|---|
| 908-989-0726 | 908-981-5560 |
| Email: [email protected] | Email: [email protected] |
Sanofi Delivers Solid Sales and Business EPS Growth in Q2 2015
Paris, July 30, 2015 / PRNewswire / — Sanofi (NYSE: SNY; EURONEXT: SAN)
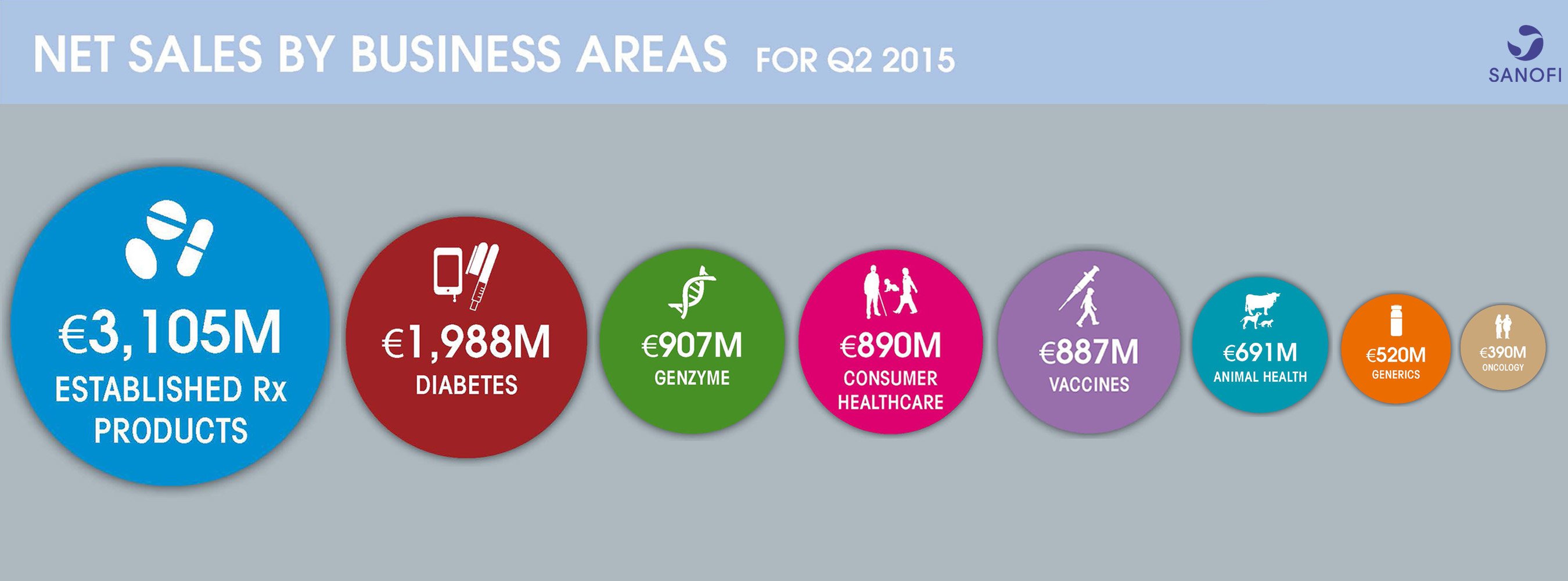
| Q2 2015 | Change (reported) | Change (CER) |
|
| Net sales | €9,378m | +16.1% | +4.9% |
| Business net income(1) | €1,840m | +19.7% | +4.2% |
| Business EPS(2) | €1.41 | +20.5% | +5.1% |
In order to facilitate an understanding of operational performance, Sanofi comments on the business net income statement. Business net income(1) is a non-GAAP financial measure. (2)(EPS) Earnings Per Share
Sanofi Chief Executive Officer, Olivier Brandicourt commented:
“In the second quarter, Sanofi delivered solid growth on both the top and bottom lines that was consistent with our expectations. We continue to execute on multiple product launches and are excited about the recent approval of Praluent®. We are also investing in our commercial infrastructure, biologic capabilities and R&D programs including in immuno-oncology. Recently, we announced a new organizational structure which will be implemented beginning in January 2016 and will simplify and focus Sanofi to optimize future growth.”
Sales growth broad based across geographies and businesses
- Group sales increased 4.9% (+16.1% on a reported basis) to €9,378 million
- Slightly lower Diabetes sales (-3.8%) consistent with previous quarter and significant U.S. market access already granted for Toujeo®
- Genzyme delivered 26.6% growth; rare disease products increased 9.1% and the Multiple Sclerosis franchise sales more than doubled
- Animal Health recorded another strong quarter (+14.2%) driven by NexGard®
- Vaccines sales grew 8.6% led by influenza and booster vaccines
- Emerging Markets sales increased 7.5%
Strong financial performance
- Business net income grew 4.2% at CER (+19.7% on a reported basis) to €1,840 million
- Business EPS increased 5.1% at CER to €1.41 and grew 20.5% on a reported basis
Significant progress in advancing innovative products
- Praluent® approved on July 24, in the U.S. and recommended for approval in the EU
- Positive Phase III topline results announced for LixiLan in diabetes in July and sarilumab in rheumatoid arthritis in May
- Phase IIIb ELIXA study results demonstrated cardiovascular safety of lixisenatide supporting the U.S. regulatory filing
- Olipudase alfa for Niemann-Pick type B advanced to Phase II and Breakthrough Therapy designation granted by FDA
New strategic collaboration with Regeneron in immuno-oncology
- Collaboration includes a PD-1 inhibitor in Phase I and other immuno-oncology antibodies currently in Preclinical development, including LAG3, GITR and PD-L1
R&D Update
Regulatory updates since the publication of the first quarter results on April 30, 2015 include the following:
- In late July, a New Drug Application (NDA) for lixisenatide, a once-daily GLP-1 analogue, was submitted to the U.S. Food and Drug Administration (FDA) for the treatment of Diabetes.
- In July, the U.S. FDA approved Praluent® (alirocumab, collaboration with Regeneron), the first FDA-approved treatment in a new class of drugs known as PCSK9 inhibitors. Praluent® is indicated as an adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease, who require additional lowering of low-density lipoprotein (LDL) cholesterol. In parallel, the CHMP also issued an opinion recommending the approval of Praluent® in EU in July. The final EMA decision is expected in late September.
- In July, the Ministry of Health, Labor and Welfare (MHLW) in Japan granted a marketing authorization for Lantus® XR (known as Toujeo® in the U.S. and Europe), a next-generation basal insulin for the treatment of type 1 and type 2 diabetes. Toujeo® was also approved in Canada in May and Australia in June.
- In June, the FDA granted Breakthrough Therapy designation to olipudase alfa. This enzyme replacement therapy is being investigated by Genzyme for the treatment of patients with Niemann-Pick disease Type B.
At the end of July 2015, the R&D pipeline contained 36 pharmaceutical new molecular entities (excluding Life Cycle Management) and vaccine candidates in clinical development of which 12 are in Phase III or have been submitted to the regulatory authorities for approval.
2015 Guidance
- The performance of the second quarter is in line with the full year guidance announced on February 5, 2015. Sanofi expects 2015 Business EPS(2) to be stable to slightly growing versus 2014 at constant average exchange rates, barring major unforeseen adverse events.
- In addition, the positive currency impact on 2015 full-year business EPS is estimated to be approximately +10%, under the assumption that exchange rates remain stable in the following two quarters at the average rates of June 2015.
To access the full press release of the Q2 2015 results, please click here.
Forward-Looking Statements
This press release contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. These statements include projections and estimates and their underlying assumptions, statements regarding plans, objectives, intentions and expectations with respect to future financial results, events, operations, services, product development and potential, and statements regarding future performance. Forward-looking statements are generally identified by the words “expects”, “anticipates”, “believes”, “intends”, “estimates”, “plans” and similar expressions. Although Sanofi’s management believes that the expectations reflected in such forward-looking statements are reasonable, investors are cautioned that forward-looking information and statements are subject to various risks and uncertainties, many of which are difficult to predict and generally beyond the control of Sanofi, that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include among other things, the uncertainties inherent in research and development, future clinical data and analysis, including post marketing, decisions by regulatory authorities, such as the FDA or the EMA, regarding whether and when to approve any drug, device or biological application that may be filed for any such product candidates as well as their decisions regarding labelling and other matters that could affect the availability or commercial potential of such product candidates, the absence of guarantee that the product candidates if approved will be commercially successful, the future approval and commercial success of therapeutic alternatives, the Group’s ability to benefit from external growth opportunities, trends in exchange rates and prevailing interest rates, the impact of cost containment policies and subsequent changes thereto, the average number of shares outstanding as well as those discussed or identified in the public filings with the SEC and the AMF made by Sanofi, including those listed under “Risk Factors” and “Cautionary Statement Regarding Forward-Looking Statements” in Sanofi’s annual report on Form 20-F for the year ended December 31, 2014. Other than as required by applicable law, Sanofi does not undertake any obligation to update or revise any forward-looking information or statements.
About Sanofi
Sanofi, a global healthcare leader, discovers, develops and distributes therapeutic solutions focused on patients’ needs. Sanofi has core strengths in diabetes solutions, human vaccines, innovative drugs, consumer healthcare, emerging markets, animal health and Genzyme. Sanofi is listed in Paris (EURONEXT: SAN) and in New York (NYSE: SNY).
Sanofi is the holding company of a consolidated group of subsidiaries and operates in the United States as Sanofi US. For more information on Sanofi US, please visit http://www.sanofi.us or call 1-800-981-2491.