Abbott Announces CE Mark for New Advancement of Absorb Stent System for People with Heart Disease
- More Than 100,000 People with Coronary Artery Disease Have Been Treated with Absorb
- Abbott on Track to Submit for Regulatory Approvals of Absorb in the U.S., Japan and China
“Absorb GT1 will enable physicians to treat more people with coronary artery disease due to its improved delivery system,” said Christoph Kurt Naber, director of Contilia Heart and Vascular Centre, Essen, Germany. “Dissolving stents are the next logical step in the treatment of patients with coronary artery disease as these stents completely dissolve after opening up clogged arteries and restoring blood flow. With the prevalence of CAD around the world, this therapy has the potential to improve the health of many people.”
Last year, Abbott announced positive one-year clinical results from ABSORB II, the world’s first prospective, randomized, controlled trial comparing the safety and effectiveness of the fully dissolving Absorb heart device to Abbott’s market-leading, metallic XIENCE family of drug eluting stents. At one year, overall clinical outcomes for Absorb were comparable to XIENCE. The trial, conducted primarily in Europe, included 501 people with CAD. At EuroPCR, a scientific meeting for cardiologists held in Paris, Absorb data will be presented throughout the conference, which runs from May 19-21, 2015. EuroPCR is the official congress of the European Association of Percutaneous Cardiovascular Interventions, with the goal of reducing the burden of cardiovascular disease.
“Coronary artery disease is the most common disease in developed countries, and the new Absorb GT1 catheter delivery system may improve the ability of doctors to treat more people with CAD by opening up coronary blockages in hard-to-reach areas with this novel, fully dissolving stent,” said Charles Simonton, M.D., FACC, FSCAI, chief medical officer and divisional vice president, Medical Affairs, vascular, Abbott. “Abbott is committed to developing scientific and technological innovations that help people regain their health and get back to their lives, and this latest Absorb advancement will allow more people to benefit from this breakthrough medical technology.”
Absorb is currently available in more than 70 countries worldwide. The company recently completed its submission for regulatory approval of Absorb in Japan, and it plans to submit reports, including data from pivotal trials, for regulatory approvals in the United States and China in the coming months. Combined, these three countries represent more than 50 percent of the world’s heart stent procedures. Currently, Absorb is an investigational device in the U.S. and is not approved for U.S. commercial use.
About the Absorb Bioresorbable Vascular Scaffold (BVS)
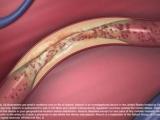
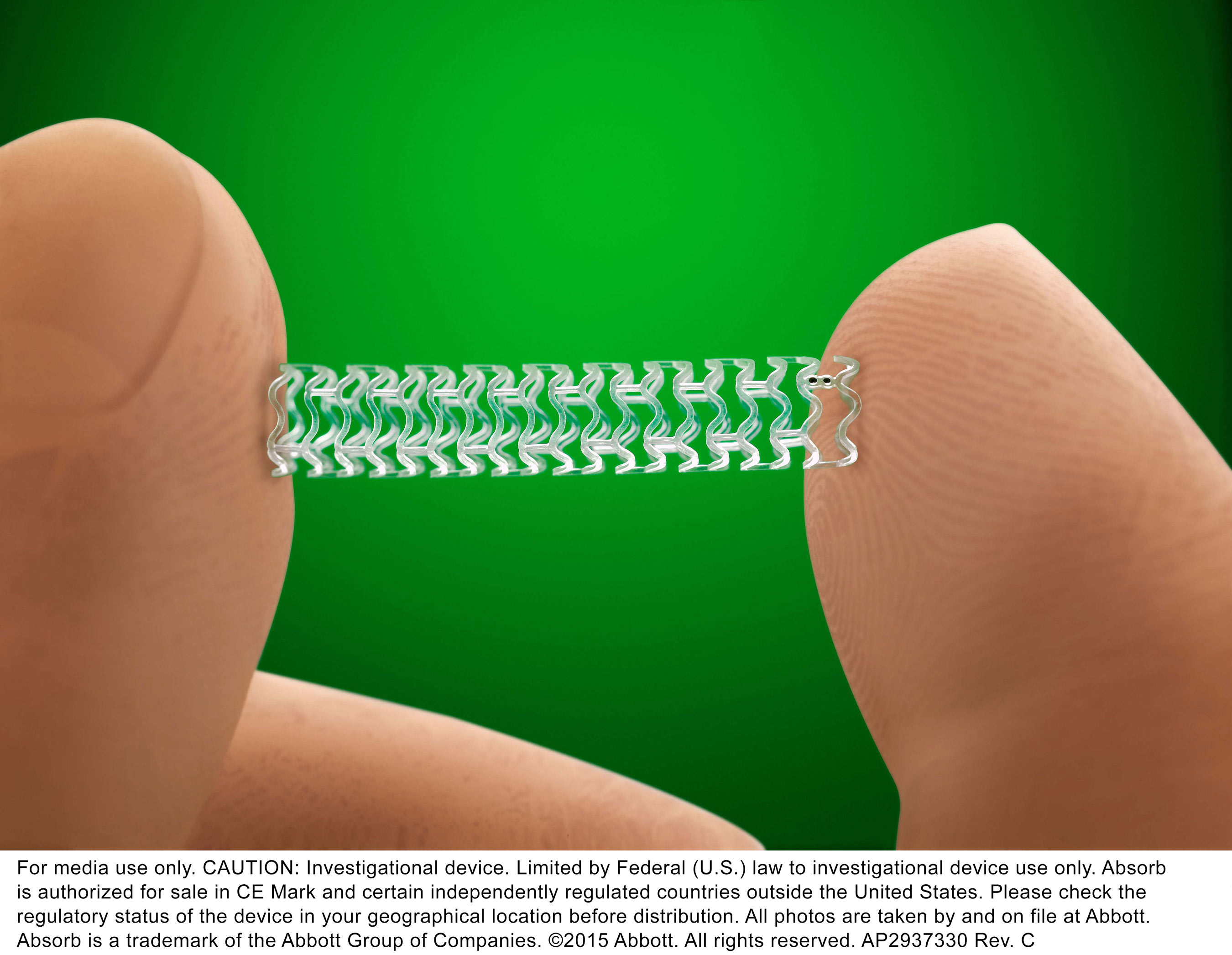
Absorb is a first-of-its-kind device that functions like a metallic stent by opening a blocked artery in the heart and restoring blood flow. However, unlike a metallic stent, which cages the vessel, Absorb is more flexible and dissolves over time, leaving behind a treated vessel free of a permanent implant with the potential to flex, pulse and dilate in response to various demands on the heart, based on people’s lifestyle and activities, such as exercise.1,2
Abbott’s BVS delivers everolimus, an anti-proliferative drug used in Abbott’s XIENCE coronary stent systems. Everolimus was developed by Novartis Pharma AG and is licensed to Abbott by Novartis for use on its drug eluting vascular devices. Everolimus has been shown to inhibit in-stent neointimal growth in the coronary vessels following stent or scaffold implantation.
About Coronary Artery Disease
Heart disease is the leading cause of death for men and women around the world, and coronary artery disease is the most common type of heart disease.3,4 Coronary artery disease occurs when arteries that supply blood to the heart become narrowed or blocked due to plaque buildup, leading to chest pain or shortness of breath and increased risk of heart attack.
About Abbott
Abbott is a global healthcare company devoted to improving life through the development of products and technologies that span the breadth of healthcare. With a portfolio of leading, science-based offerings in diagnostics, medical devices, nutritionals and branded generic pharmaceuticals, Abbott serves people in more than 150 countries and employs approximately 73,000 people.
Visit Abbott at www.abbott.com and connect with us on Twitter at @AbbottNews.
###
1 Absorb completely dissolves except for two pairs of tiny metallic markers, which help guide placement and remain in the artery to enable a physician to see where the device was placed.
2 Preliminary evidence suggests that natural vessel function is possible with Absorb and may improve long term outcomes.
3 The top 10 causes of death, World Health Organization. June 2011
Available at: http://www.who.int/mediacentre/factsheets/fs310/en/index.html
4 Coronary Artery Disease. National Heart, Lung and Blood Institute. May 2011
Available at: http://www.nhlbi.nih.gov/health/health-topics/topics/cad/
Abbott Media
Steve Kelly
(408) 845-3427
Mira Jang
(408) 250-5782
Abbott Financial
Tina Ventura
(224) 668-7606
Video
Photo Gallery
Download
Download Absorb AnimationInfographic