Today, the Pharmaceutical Research and Manufacturers of America (PhRMA) released a new report, “A Decade of Innovation in Rare Diseases,” to document the significant progress made in the last 10 years in understanding a broad range of rare diseases and translating this knowledge into groundbreaking therapies for a variety of patient populations.
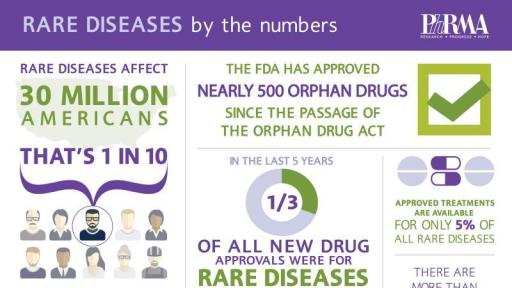
The report illustrates that more than 230 new medicines to treat rare or “orphan” diseases were approved by the U.S. Food and Drug Administration (FDA) in the last decade, and there are currently more than 450 orphan drugs in development.
It also explores significant treatment advances seen over the past decade in five rare diseases which have led to improvements in patient survival and quality of life: chronic myelogenous leukemia (CML), chronic lymphocytic leukemia (CLL), pulmonary arterial hypertension (PAH), hereditary angioedema (HAE), and cystic fibrosis (CF). Furthermore, the report spotlights additional rare conditions where major milestones transformed treatment —including several in which first–ever treatment options have become available to patients.
Multimedia
Key findings include:
- Targeted therapies can now effectively treat many of the recently identified mutated forms of CML and allow for treatment plans tailored to each patient’s particular genetic profile.
- Novel targeted therapies treat the root cause of CLL, resulting in lasting remissions and new treatment options for even the sickest patients.
- New treatments go beyond symptom management to treat the underlying cause of PAH, allowing patients to maintain active lifestyles with reduced risk of serious heart events.
- New discoveries in the underlying cause of HAE have led to breakthroughs in both preventative and acute treatment options for patients, including those targeting the root cause of the disease.
- CF patients now have new treatment options allowing for better management of symptoms as well as a new option which allows many to target the underlying cause of their disease. If mortality rates continue to decline, patients may now hope to live into their 50s.
“Rare diseases are one of the most scientifically complex health challenges we face,” said PhRMA President and CEO John J. Castellani. “Over the last 10 years, biopharmaceutical researchers have leveraged cutting-edge technologies and dramatically expanded our understanding of rare diseases to develop new therapies for chronic myelogenous leukemia, cystic fibrosis, and other diseases which often impact very small patient populations. We continue to work toward options for rare disease patients, where there may currently be few or no treatments available.”
The new report was released ahead of Rare Disease Day, which will be celebrated on Saturday, February 28, 2015. The day is an opportunity for the international rare disease community – including academia, patient advocacy groups, pharmaceutical and biotechnology companies, research and regulatory government agencies – to collectively educate and raise awareness about rare diseases and their impact on the lives of patients and their families.
Thirty million Americans, or one in ten, and an estimated 350 million people worldwide are currently living with a rare disease, which is defined as a condition that affects fewer than 200,000 people. There are currently 7,000 known rare diseases and approximately 80 percent are caused by genetic abnormalities. However, despite incredible progress made against rare disease in recent years, only 5 percent have available treatment options.
While there remains a significant need to develop new medicines for patients, this is a challenging endeavor. Rare diseases are often complex and the underlying biological mechanisms that cause them are not always well understood. Nevertheless, researchers continue to build on recent breakthroughs and are dedicated to bringing new medicines to patients. With more than 450 medicines in development to treat rare disease, the promise has never been greater for patients.
For more information on rare diseases, view the “Medicines in Development for Rare Diseases” report.
###
About PhRMA
The Pharmaceutical Research and Manufacturers of America (PhRMA) represents the country’s leading innovative biopharmaceutical research companies, which are devoted to discovering and developing medicines that enable patients to live longer, healthier, and more productive lives. Since 2000, PhRMA member companies have invested more than $550 billion in the search for new treatments and cures, including an estimated $51.1 billion in 2013 alone.
Connect with PhRMA
For information on how innovative medicines save lives, please visit:
www.PhRMA.org
www.FromHopetoCures.org
www.Facebook.com/PhRMA
www.Twitter.com/PhRMA