Media Relations
Mary Kathryn Steel
908-989-0726
Email: [email protected]
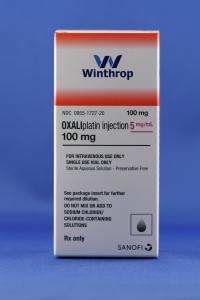
Sanofi Launches Authorized Generic Version of Eloxatin® (oxaliplatin injection)
Generics Division of Sanofi, Winthrop US, to Market the Drug
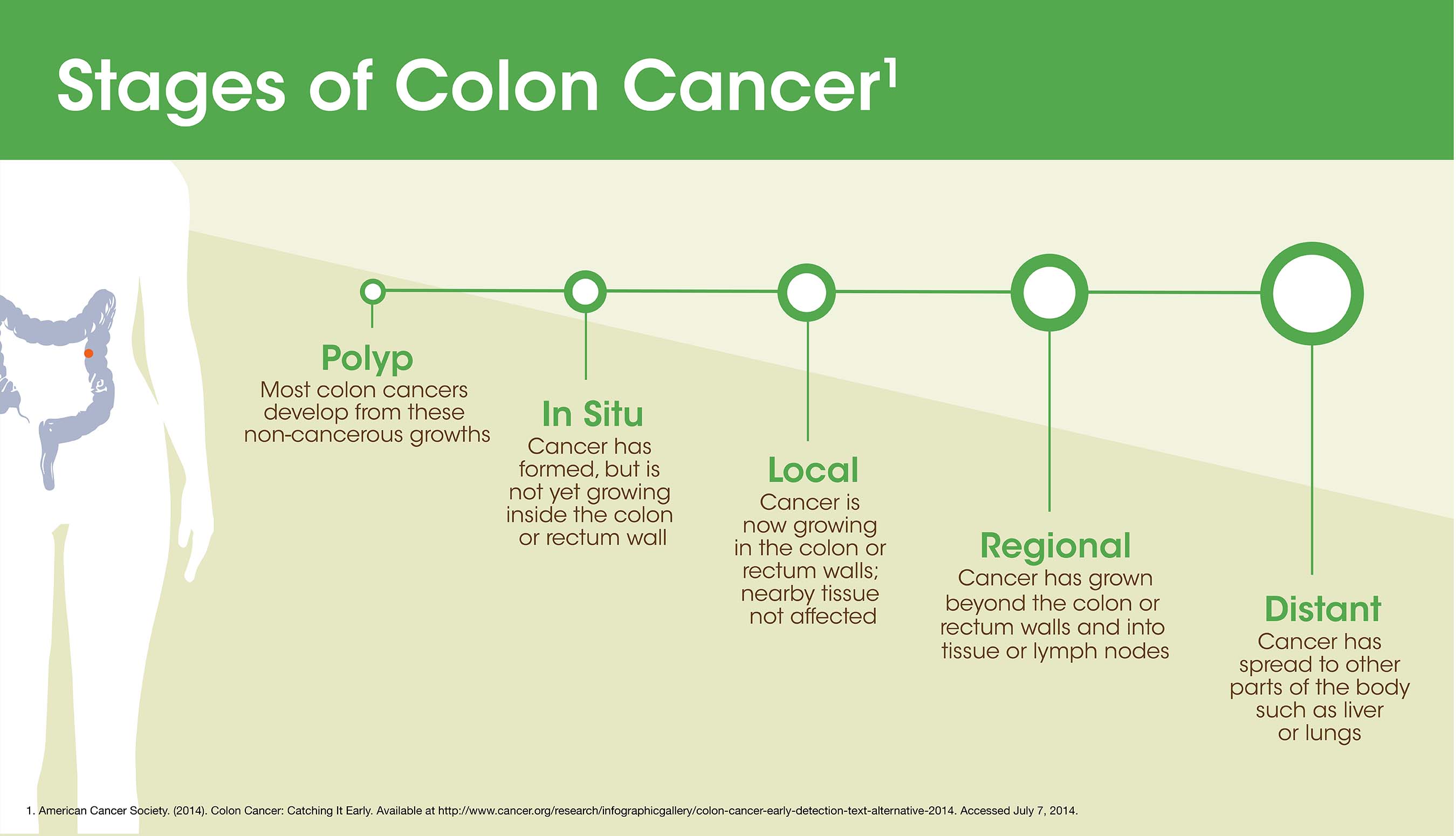
Eloxatin is a platinum–based drug used in combination with infusional 5–fluorouracil/leucovorin. This treatment is indicated for treatment of advanced colorectal cancer or as adjuvant treatment of stage III colon cancer in patients who have undergone complete resection of the primary tumor. The authorized generic version of Eloxatin will be available in the same sizes, 50 mg and 100 mg single–use vials.
“We are committed to broadening access to our medicines and providing more affordable treatment options for patients,” said Charles Hugh-Jones, MD, FRCP, Chief Medical Officer, North America, Sanofi. “The availability of an authorized generic version of Eloxatin is welcomed news for patients and physicians alike.”
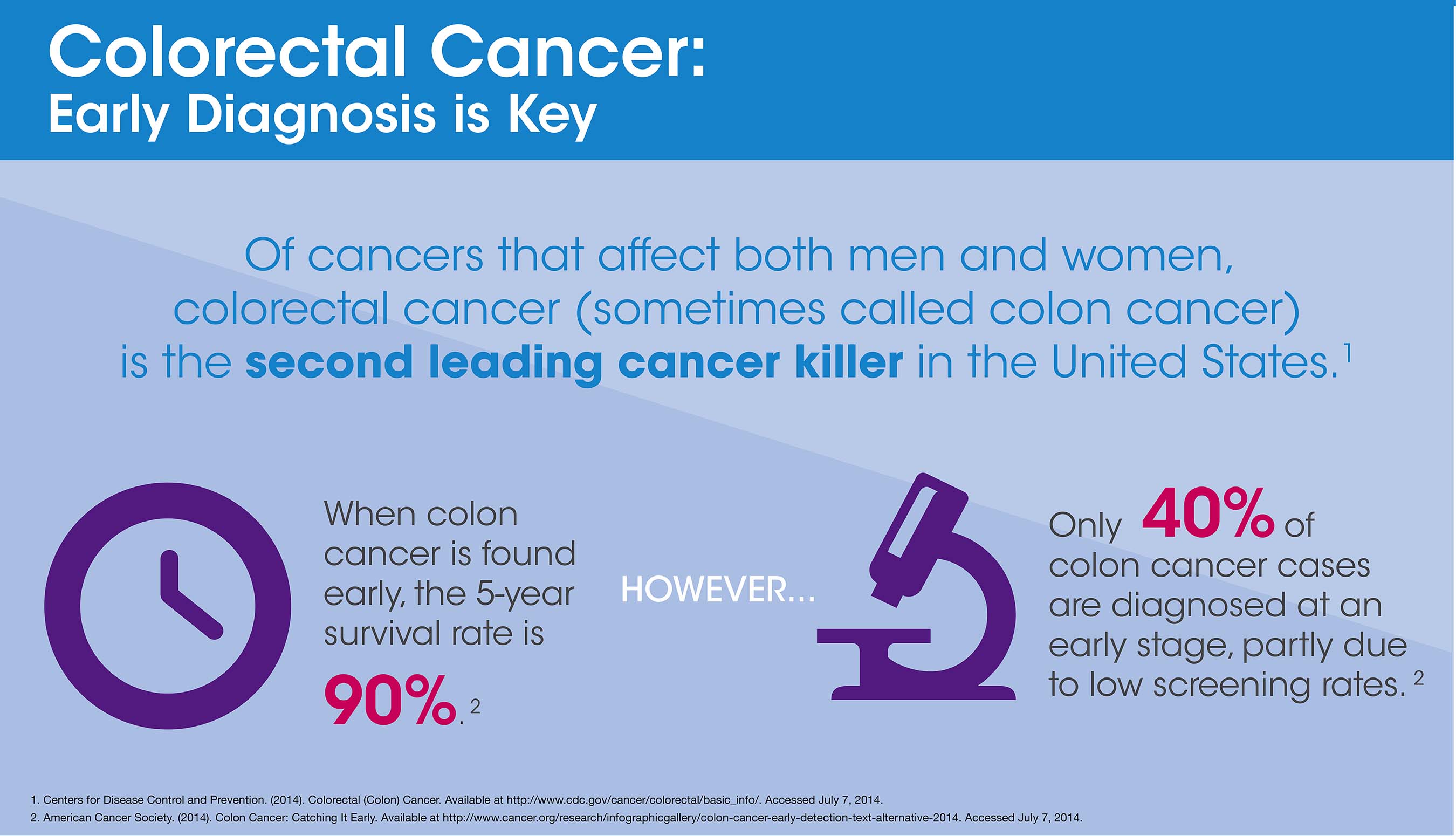
According to the Centers for Disease Control and Prevention (CDC), “of cancers that affect both men and women, colorectal cancer is the second leading cancer killer in the United States.”1
Winthrop US delivers affordable solutions to the healthcare community by transforming Sanofi’s branded products into authorized generics and is devoted to supplying high quality products to its customers with excellent service.
Important Safety Information for Eloxatin® (oxaliplatin injection)
ELOXATIN can cause serious allergic reactions, including allergic reactions that may cause death. ELOXATIN is a platinum–based medicine. Serious allergic reactions including death can occur in people who take ELOXATIN and who have had previous allergic reactions to platinum medicines. Serious allergic reaction can happen within a few minutes of your infusion or any time during your treatment with ELOXATIN.
- Do not take ELOXATIN if you are allergic to any of the ingredients in Eloxatin or other medicines that contain platinum. Tell your doctor right away if you feel like your throat is closing up, have shortness of breath, a flushed face, a rash, itching/hives, swelling of lips or tongue, sudden cough, dizziness, sweating or chest pain
- ELOXATIN can cause nerve problems. Tell your doctor right away if you become sensitive to cold temperatures and cold objects; have trouble breathing, swallowing or saying words; experience jaw tightness, odd feelings in your tongue, or chest pressure; pain; tingling or burning in your hands, feet or around your mouth or throat.
- ELOXATIN can cause a rare condition that affects the brain called Reversible Posterior Leukoencephalopathy, also known as RPLS. Tell your doctor right away if you have any of the following signs and symptoms of RPLS: headache, confusion or a change in the way you think, seizures, or vision problems, such as blurriness or vision loss. You should not operate heavy machines, or engage in dangerous activities if you have vision problems while receiving ELOXATIN.
- Tell your doctor if you develop dry cough or have trouble breathing; these may be signs of serious lung disease.
- ELOXATIN can cause liver problems (hepatotoxicity); your doctor will do blood tests to watch for this.
- Tell your doctor if you have any kidney problems before receiving ELOXATIN because this could change the amount of ELOXATIN which you receive for your treatment.
- Because of the potential risk of fetal harm, pregnant women should not receive ELOXATIN. Women of childbearing potential should avoid becoming pregnant while receiving ELOXATIN.
- ELOXATIN can cause a decrease in white blood cells, which can lead to infections. Tell your doctor right away if you develop any of the following signs of infection: fever, chills, or shivering; cough that brings up mucus; burning or pain on urination; pain on swallowing; sore throat; or redness or swelling at injection site.
- The most common side effects of ELOXATIN include a decrease in white blood cells, red blood cells and platelets, infection, high blood pressure, nausea, constipation, stomach pain, decreased appetite, hair loss, vomiting, injection site reactions, mouth sores, diarrhea, tiredness and bleeding or bruising. Tell your doctor about any signs or symptoms of bleeding or bruising.
- Tell your doctor about all the medications you take, including prescription and non–prescription medications, vitamins and herbal supplements.
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of ELOXATIN. For more information, ask your doctor or pharmacist.
Please click here for full Prescribing Information for Eloxatin® (oxaliplatin injection), including Boxed WARNING, and visit: http://products.sanofi.us/eloxatin/eloxatin.html
About Sanofi
Sanofi, a global healthcare leader, discovers, develops and distributes therapeutic solutions focused on patients’ needs. Sanofi has core strengths in the field of healthcare with seven growth platforms: diabetes solutions, human vaccines, innovative drugs, consumer healthcare, emerging markets, animal health and the new Genzyme. Sanofi is listed in Paris (EURONEXT: SAN) and in New York (NYSE: SNY).
Forward Looking Statements
This press release contains forward–looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward–looking statements are statements that are not historical facts. These statements include projections and estimates and their underlying assumptions, statements regarding plans, objectives, intentions and expectations with respect to future financial results, events, operations, services, product development and potential, and statements regarding future performance. Forward–looking statements are generally identified by the words “expects”, “anticipates”, “believes”, “intends”, “estimates”, “plans” and similar expressions. Although Sanofi’s management believes that the expectations reflected in such forward–looking statements are reasonable, investors are cautioned that forward–looking information and statements are subject to various risks and uncertainties, many of which are difficult to predict and generally beyond the control of Sanofi, that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward–looking information and statements. These risks and uncertainties include among other things, the uncertainties inherent in research and development, future clinical data and analysis, including post marketing, decisions by regulatory authorities, such as the FDA or the EMA, regarding whether and when to approve any drug, device or biological application that may be filed for any such product candidates as well as their decisions regarding labelling and other matters that could affect the availability or commercial potential of such product candidates, the absence of guarantee that the product candidates if approved will be commercially successful, the future approval and commercial success of therapeutic alternatives, the Group’s ability to benefit from external growth opportunities, trends in exchange rates and prevailing interest rates, the impact of cost containment policies and subsequent changes thereto, the average number of shares outstanding as well as those discussed or identified in the public filings with the SEC and the AMF made by Sanofi, including those listed under “Risk Factors” and “Cautionary Statement Regarding Forward–Looking Statements” in Sanofi’s annual report on Form 20–F for the year ended December 31, 2013. Other than as required by applicable law, Sanofi does not undertake any obligation to update or revise any forward–looking information or statements.
1Centers for Disease Control and Prevention. Basic information about colorectal cancer. Available at: http://www.cdc.gov/cancer/colorectal/basic_info/. Accessed July 28, 2014.