Usage
Use
DUOPA (carbidopa and levodopa) enteral suspension is a treatment for motor fluctuations in patients with advanced Parkinson’s disease.
Important Safety Information about DUOPA (carbidopa and levodopa) enteral suspension
DUOPA (carbidopa and levodopa) enteral suspension is a prescription medicine used for treatment of advanced Parkinson's disease. DUOPA contains 2 medicines: carbidopa and levodopa.
DUOPA is given over a period of 16 hours by a pump through a tube that requires a small hole (stoma) into the stomach. Before the procedure, a discussion with a healthcare provider about any previous procedures or problems with the abdomen area is required. Risks of the procedure may result in blockage of the stomach or intestine, stopping of movement through the intestine, infection, inflammation of the pancreas, stomach pain, gas, stomach and intestinal ulcers or bleeding, nausea, blocking of the tube, or other serious outcomes that may lead to surgery or be fatal.
DUOPA should not be taken by people who are currently taking or have recently taken (within 2 weeks) a medicine called a nonselective monoamine oxidase (MAO) inhibitor. Serious side effects of medicines that contain carbidopa and levodopa, including DUOPA, include sleepiness or suddenly falling asleep without warning during daily activities. Patients should not drive or operate heavy machinery until they are sure how DUOPA affects them. Some patients taking Parkinson's disease medications, including DUOPA, can experience low blood pressure; fast, irregular heartbeat or chest pain; dizziness or fainting; hallucinations or confusion; intense urges they are unable to control, including the urge to gamble, spend money, or overeat; increased sexual urges; and other intense urges. DUOPA can cause or worsen depression. Patients should be counseled to report symptoms of depression or thoughts of suicide. Suddenly stopping DUOPA or rapid dose reduction can result in fever and confusion. Taper dose and monitor patients for fever, confusion, or severe muscle stiffness. Progressive weakness or loss of sensation in the fingers or feet may occur. People with Parkinson's disease have a greater risk of melanoma than the general population and should be monitored while on DUOPA. Worsening of glaucoma may occur. The most common adverse reactions (>7% and greater than carbidopa and levodopa immediate release) were complication of device insertion, nausea, constipation, incision site erythema, dyskinesia, depression, post procedural discharge, peripheral edema, hypertension, upper respiratory tract infection, oropharyngeal pain, atelectasis, confusional state, anxiety, dizziness, and hiatal hernia.
AbbVie Announces U.S. FDA Approval of DUOPA™ (carbidopa and levodopa) Enteral Suspension for the Treatment of Motor Fluctuations in Patients with Advanced Parkinson’s Disease
- DUOPA is the first and only treatment providing 16 continuous hours of carbidopa and levodopa for motor fluctuations in advanced Parkinson’s disease
- In a clinical trial, patients treated with DUOPA experienced significantly greater improvement in “off” time than patients treated with oral carbidopa–levodopa immediate release tablets
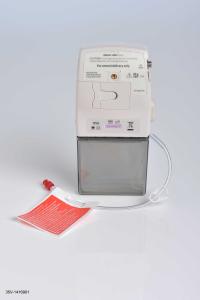
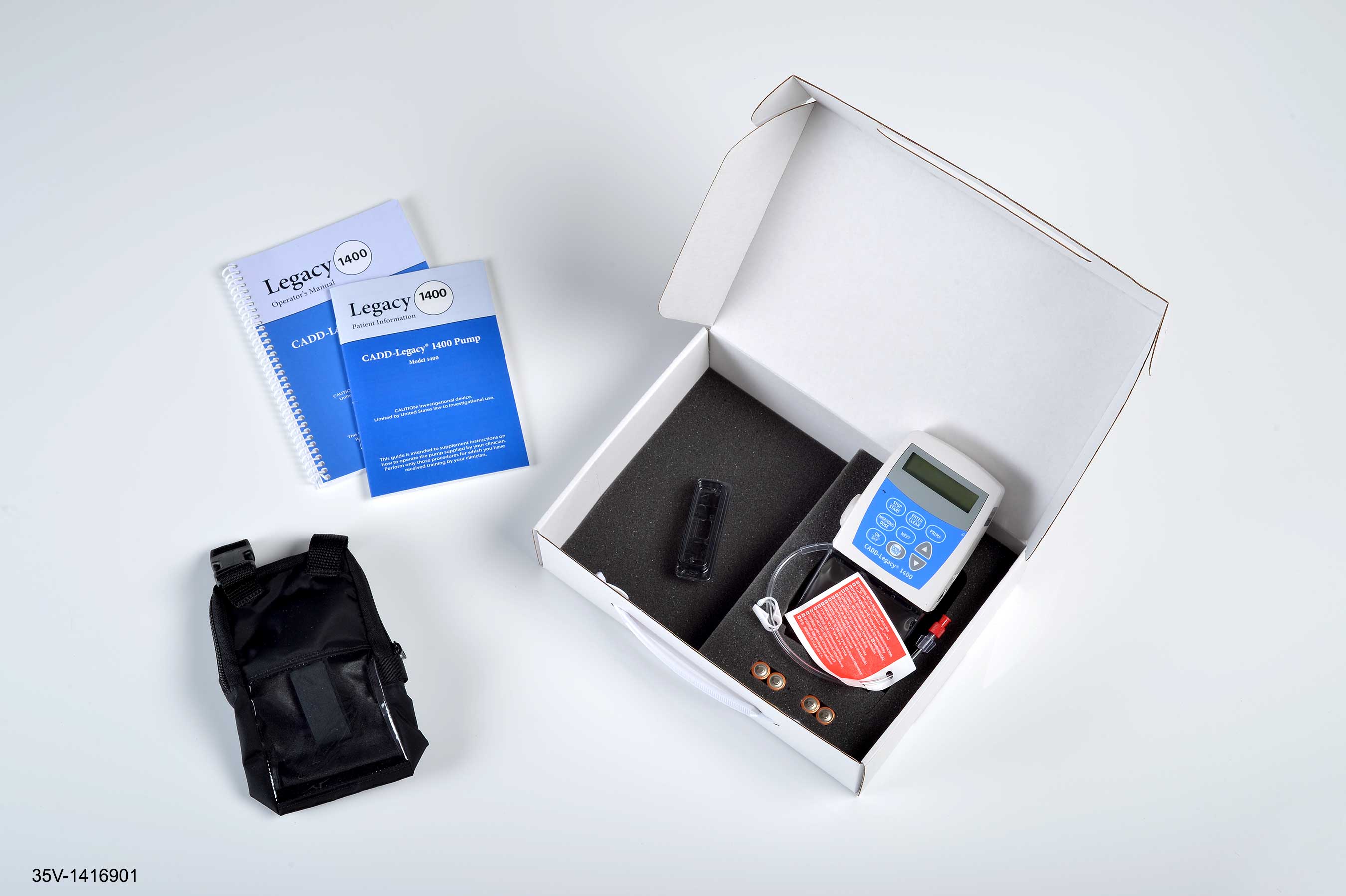
NORTH CHICAGO, Ill. January 12, 2015 – The U.S. Food and Drug Administration (FDA) has approved AbbVie’s (NYSE: ABBV) DUOPA™ (carbidopa and levodopa) enteral suspension for the treatment of motor fluctuations for people with advanced Parkinson’s disease. DUOPA is administered using a small, portable infusion pump that delivers carbidopa and levodopa directly into the small intestine for 16 continuous hours via a procedurally–placed tube.
DUOPA was approved by the FDA as an orphan drug, a designation granted to products intended for the treatment of rare diseases or conditions affecting fewer than 200,000 patients in the U.S.
“There is unmet need for treatment options for patients with advanced Parkinson’s disease. As the disease advances, it can be difficult to control motor features,” said C. Warren Olanow, M.D., Professor, Department of Neurology and Department of Neuroscience, Mount Sinai School of Medicine, and lead investigator of the DUOPA pivotal trial. “In clinical trials, DUOPA was shown to significantly reduce the amount of off time advanced Parkinson’s disease patients experienced.”
In the advanced stages of Parkinson’s disease, patients may begin to experience “off” time, or periods of poor mobility, slowness and stiffness. Additionally, in Parkinson’s disease patients, the spontaneous emptying of the stomach becomes delayed and unpredictable, which can affect the timing of when orally administered medicines leave the stomach and are absorbed in the small intestine. DUOPA provides patients with the same active ingredients as orally–administered carbidopa and levodopa immediate release, but is delivered in a suspension that goes directly into the small intestine via a tube placed by a percutaneous endoscopic gastrostomy procedure with jejunal extension (PEG–J). This type of administration is intended to bypass the stomach.
“The FDA approval of DUOPA is another significant milestone for AbbVie’s pipeline,” said Michael Severino, M.D., Executive Vice President, Research and Development and Chief Scientific Officer, AbbVie. “This advancement is important for patients with advanced Parkinson’s disease and their care teams, as it provides a new therapeutic option to help manage motor symptoms.”
“Due to the progressive nature of Parkinson's disease, it can be difficult to treat over time, especially in the advanced stages,” said Joyce Oberdorf, President and CEO, National Parkinson Foundation. “Our organization is encouraged by the introduction of a new therapy that may provide another treatment option for affected patients and families.”
About the Duopa Clinical Trial Program
The DUOPA approval is based on a Phase 3, 12–week, double–blind, double-placebo, active control, parallel group, multi–center trial (N=71) that compared the efficacy and safety of DUOPA to oral, immediate-release (IR) carbidopa-levodopa tablets in advanced Parkinson’s disease patients. The study showed that DUOPA significantly reduced daily (per 16 waking hours) mean “off” time at 12 weeks by four hours, which resulted in an average of 1.9 fewer hours of “off time” when compared to carbidopa–levodopa IR tablets. Treatment with DUOPA was also associated with an improved mean “on” time (periods when the medication is working and symptoms are controlled) without troublesome dyskinesia (uncontrolled movement that does not interfere with normal daily activities) by four hours at 12 weeks, which resulted in an average of 1.9 hours more “on” time when compared to carbidopa–levodopa IR tablets. The most common adverse events (>7% and greater than carbidopa and levodopa immediate release) were complication of device insertion, nausea, constipation, incision site erythema, dyskinesia, depression, post procedural discharge, peripheral edema, hypertension, upper respiratory tract infection, oropharyngeal pain, atelectasis, confusional state, anxiety, dizziness and hiatal hernia.
About Parkinson’s Disease
Parkinson’s disease is a progressive and chronic movement disorder1 characterized by tremor, muscle rigidity, slowness of movement and difficulty with balance.2 It is classified as a movement disorder resulting from the loss of dopamine-producing brain cells.3 The motor symptoms of Parkinson’s disease begin when approximately 60-80 percent of the dopamine-producing cells in the brain are lost and symptoms continue to worsen slowly over the course of time.4 While there is no known cure for the disease, there are treatments available to help reduce symptoms.3
As Parkinson’s disease progresses, patients may experience fluctuations from an “on” state to an “off state,” during which they are slower, stiffer and experience more difficulty moving. Patients may also experience dyskinesias (involuntary movements).
In the United States, it is estimated that 60,000 new cases of Parkinson’s disease are reported each year, adding to as many as 1 million people who currently have the disease.
AbbVie has launched a product support program, called DuoConnect™, which is intended to provide a broad range of support for DUOPA patients. The DuoConnect program can be accessed soon by calling 1–844–DUO–4YOU (1–844–386–4968).
Additionally, for people living with advanced Parkinson’s disease who face financial difficulties, the AbbVie Patient Assistance Program may provide DUOPA at no cost. A co–pay assistance program will be available for commercially–insured patients being treated with DUOPA.
AbbVie also supports independent non–profit organizations that assist eligible patients enrolled in federal and private insurance plans with their out–of–pocket medication costs.
About DUOPA (carbidopa and levodopa) enteral suspension
DUOPA is a new approach to the administration of carbidopa and levodopa for the treatment of motor fluctuations for people with advanced Parkinson’s disease. Carbidopa–levodopa is widely recognized as an effective treatment for motor fluctuations of the disease.5 DUOPA was reviewed and approved by the FDA as a combination product with use of the CADD Legacy 1400 pump.
Carbidopa and levodopa enteral suspension is currently approved in 41 countries and is marketed by AbbVie as DUODOPA® outside the United States.
Full Prescribing Information, including the Medication Guide can be found at www.rxabbvie.com.
DUOPA (carbidopa and levodopa) enteral suspension is a prescription medicine used for treatment of advanced Parkinson’s disease. DUOPA contains two medicines: carbidopa and levodopa.
DUOPA is given over a period of 16 hours by a pump through a tube that requires a small hole (stoma) into the stomach. Before the procedure, a discussion with a healthcare provider about any previous procedures or problems with the abdomen area is required. Risks of the procedure may result in blockage of the stomach or intestine, stopping of movement through the intestine, infection, inflammation of the pancreas, stomach pain, gas, stomach and intestinal ulcers or bleeding, nausea, blocking of the tube, or other serious outcomes that may lead to surgery or be fatal.
DUOPA should not be taken by people who are currently taking or have recently taken (within 2 weeks) a medicine called a nonselective monoamine oxidase (MAO) inhibitor. Serious side effects of medicines that contain carbidopa and levodopa, including DUOPA, include sleepiness or suddenly falling asleep without warning during daily activities. Patients should not drive or operate heavy machinery until they are sure how DUOPA affects them. Some patients taking Parkinson’s disease medications, including DUOPA, can experience low blood pressure; fast, irregular heartbeat or chest pain; dizziness or fainting; hallucinations or confusion; intense urges they are unable to control, including the urge to gamble, spend money, or overeat; increased sexual urges; and other intense urges. DUOPA can cause or worsen depression. Patients should be counseled to report symptoms of depression or thoughts of suicide. Suddenly stopping DUOPA or rapid dose reduction can result in fever and confusion. Taper dose and monitor patients for fever, confusion, or severe muscle stiffness. Progressive weakness or loss of sensation in the fingers or feet may occur. People with Parkinson’s disease have a greater risk of melanoma than the general population and should be monitored while on DUOPA. Worsening of glaucoma may occur. The most common adverse reactions (>7% and greater than carbidopa and levodopa immediate release) were complication of device insertion, nausea, constipation, incision site erythema, dyskinesia, depression, post procedural discharge, peripheral edema, hypertension, upper respiratory tract infection, oropharyngeal pain, atelectasis, confusional state, anxiety, dizziness, and hiatal hernia.
About AbbVie
AbbVie is a global, research-based biopharmaceutical company formed in 2013 following separation from Abbott Laboratories. The company’s mission is to use its expertise, dedicated people and unique approach to innovation to develop and market advanced therapies that address some of the world’s most complex and serious diseases. AbbVie employs approximately 25,000 people worldwide and markets medicines in more than 170 countries. For further information on the company and its people, portfolio and commitments, please visit www.abbvie.com. Follow @abbvie on Twitter or view careers on our Facebook or LinkedIn page.
###
Media:
David Freundel
[email protected]
(847) 937-4522
Gentry Lassiter
[email protected]
(847) 935-5591
Investors:
Liz Shea
[email protected]
(847) 935-2211
1Parkinson’s Disease Foundation. http://www.pdf.org/en/about_pd. Accessed January 20, 2014.
2The Michael J. Fox Foundation for Parkinson’s Research. https://www.michaeljfox.org/understanding-parkinsons/living-with-pd/topic.php?symptoms. Accessed January 20, 2014.
3The Michael J. Fox Foundation for Parkinson’s Research. https://www.michaeljfox.org/understanding-parkinsons/i-have-got-what.php#q2. Accessed January 20, 2014.
4National Parkinson Foundation. http://www.parkinson.org/Parkinson-s-Disease/PD-101/What-is-Parkinson-s-disease. Accessed January 20, 2014.
5The Michael J. Fox Foundation for Parkinson’s Research. https://www.michaeljfox.org/understanding-parkinsons/living-with-pd/topic.php?medication. Accessed January 28, 2014.
Duopa B-Roll

Related Documents
Full Prescribing InformationImportant Safety Facts for DUOPA (carbidopa and levodopa) enteral suspension
Duopa Fact Sheet