The New Contour®Next One Blood Glucose Monitoring System from Ascensia Diabetes Care Effortlessly Logs Blood Glucose Readings to Help People with Diabetes Easily Understand Their Results
Newbury, United Kingdom, 19 October 2016 – Ascensia Diabetes Care today announced the availability of the CONTOUR®NEXT ONE blood glucose monitoring system (BGMS) in the United Kingdom, one of the next steps in the evolution of self-monitoring of blood glucose. As part of the system, the CONTOUR®NEXT ONE meter and CONTOUR®DIABETES app seamlessly connect, which makes capturing blood glucose results effortless for people with diabetes. The system enables patterns and trends to be revealed that can help people understand their diabetes, and may help to improve diabetes management.
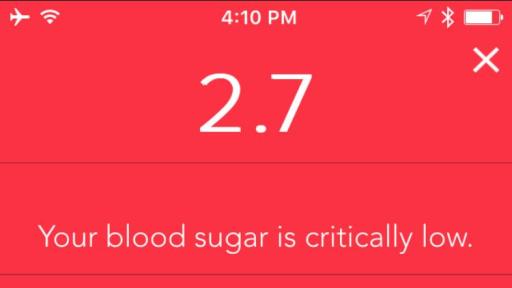
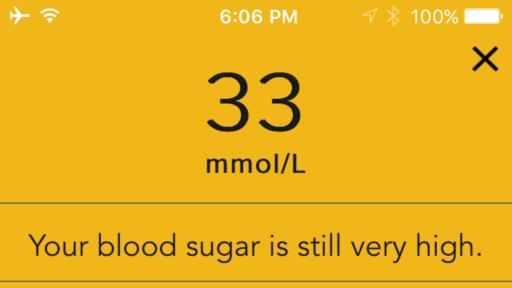
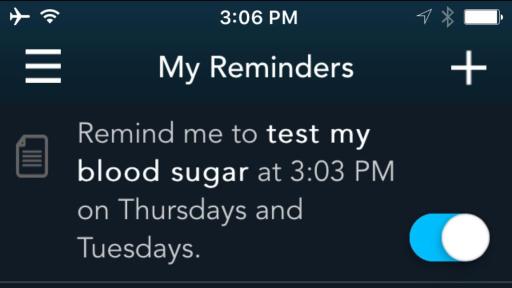
The system features an easy-to-use wireless-enabled smart meter that links to a smart mobile device via Bluetooth® connectivity. The CONTOUR®NEXT ONE meter gives immediate feedback and also seamlessly connects to the CONTOUR®DIABETES app, which collects, stores and analyses patient blood glucose results received from the meter. The app combines these results with other data recorded by the user, to provide detailed information about their condition that can help them understand how their everyday lives may impact their blood glucose readings, including alerts for critical high or critical low readings. Through the app, meter users can also share with their healthcare professional the reports of their blood glucose results and patterns, either in advance or on the day of their appointment, helping to create more informed discussions.
“People with diabetes are turning to mobile devices to help manage and track their condition,” said Dr. Partha Kar, Consultant in Diabetes and Endocrinology, Portsmouth Hospitals NHS Trust. “They want solutions that seamlessly integrate into their lives and present their readings in a simple and intuitive way. The CONTOUR®NEXT ONE system appears to be a development for interconnected diabetes management that has the potential to engage more people with diabetes in their treatment plans which could lead to better outcomes”.
“The launch of the CONTOUR®NEXT ONE blood glucose monitoring system in the United Kingdom is the latest of our high quality solutions designed to meet the needs of the diabetes community. By seamlessly capturing data from the meter in the app, this system has the potential to benefit people with diabetes by improving the understanding of their condition and helping them to make more informed decisions about managing their diabetes. We are delighted it is now available to help people with diabetes in the United Kingdom,” explained Ros Barker, Ascensia Diabetes Care.
The meter also includes additional features specifically designed to meet patient needs, including the smartLIGHTTM feature that gives instant feedback on blood glucose readings, for better understanding of results. It also has Second-Chance™ sampling, which prompts blood to be reapplied if there is not enough the first time, helping to avoid lancing for a second time and the wasting of strips.
“In the management of diabetes, the accuracy and quality of blood glucose data from an individual is crucial. The CONTOUR®NEXT ONE blood glucose meter has demonstrated consistently remarkable levels of accuracy and precision for patients. There is significant value for doctors and nurses by having easy access to this highly accurate data, which can help to improve how patients are managed,” added Ros.
Published study data has shown the CONTOUR®NEXT ONE meter to be remarkably accurate1, exceeding EN ISO 15197:2015 accuracy criteria2 in both the laboratory and clinical setting. EN ISO 15197:2015 states that ≥95% of results must be within ±0.83 mmol/L of the reference result for samples with blood glucose concentrations ˂5.56 mmol/L or ±15% for samples with blood glucose concentrations ≥5.56 mmol/L. Data in the clinical setting showed that 99.4% of readings from subject-obtained fingertip results exceeds EN ISO 15197:2015 accuracy criteria and 95% of readings were shown to be within ±0.47 mmol/L or ±8.4% of the YSI reference results.1
An estimated 4 million people in United Kingdom have diabetes, according to Diabetes UK.* The primary goal of diabetes management is to control blood sugar levels and ensure they are kept within a normal range. When blood glucose levels get too high (hypgerlycaemia) or too low (hypoglycaemia), it can lead to serious complications and, in severe cases, even death.
The launch of the CONTOUR®NEXT ONE BGMS in the United Kingdom follows CE Mark approval that was recently received, on 17 May 2016. As it is app based, the system is designed to evolve in the future, bringing new capabilities and features to help people with diabetes manage their condition. Those interested in obtaining the CONTOUR®NEXT ONE meter should visit https://www.contournextone.co.uk/. The CONTOUR®DIABETES app is available to download in the Apple App Store (iOS) and Google Play (Android).
Notes for Editors
About Ascensia Diabetes Care
Ascensia Diabetes Care is a global specialist diabetes care company, dedicated to helping people living with diabetes. Our mission is to empower people living with diabetes through innovative solutions that simplify and improve their lives. We use our innovation and specialist expertise in diabetes to develop high quality solutions and tools that make a positive, daily difference for people with diabetes.
Home to the world renowned CONTOUR® portfolio of blood glucose monitoring systems, our products combine advanced technology with user-friendly functionality that help people with diabetes to manage their condition. We are committed to continued research, innovation and development of new products and solutions. As a trusted partner in the diabetes community, we collaborate closely with healthcare professionals and other partners to ensure our products meet the highest standards of accuracy, precision and reliability, and that we conduct our business compliantly and with integrity.
Ascensia Diabetes Care was established in 2016 through the sale of Bayer Diabetes Care to Panasonic Healthcare Holdings Co., Ltd. Ascensia Diabetes Care products are sold in more than 125 countries. Following the close of the transaction in all countries, Ascensia Diabetes Care will have around 1,700 employees and operations in 38 countries.
For further information please visit the Ascensia Diabetes Care website at: http://www.ascensia.com.
CONTOUR® Second-Chance™ sampling and smartLIGHT™ are trademarks and/or registered trademarks of Ascensia Diabetes Care Holdings AG.
The Bluetooth® word mark and logos are registered trademarks owned by Bluetooth SIG, Inc., and any use of such marks herein is under license.
For further information please contact:
Joseph Delahunty
FTI Consulting
[email protected]
Tel: +44 (0)20 3727 1000
Stephanie Burns
FTI Consulting
[email protected]
Tel: +44 (0)20 3727 1816
1. Christiansen et al. Poster presented at the 15th Annual Meeting of the Diabetes Technology Society, October 22-24, 2015, Bethesda, Maryland.
2. International Organization for Standardization (2015). In vitro diagnostic test systems—requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus (EN ISO 15197:2015)
* https://www.diabetes.org.uk/About_us/News/Number-of-people-with-diabetes-reaches-over-4-million/