Johnson & Johnson Launches Heartline™, the First-of-Its-Kind, Virtual Study Designed to Explore if a New iPhone App and Apple Watch can Help Reduce the Risk of Stroke
Johnson & Johnson, in collaboration with Apple, is offering eligible U.S. adults 65 years and older the opportunity to join the clinical study by downloading the Heartline Study app on iPhone
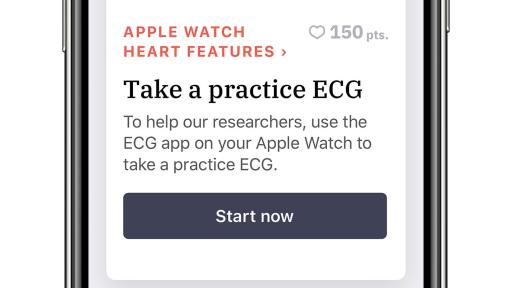
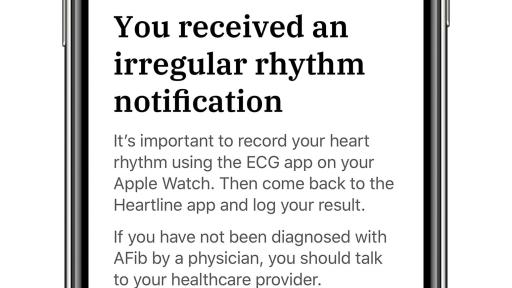
Study aims to assess whether the Heartline Study app on iPhone, and the ECG app and irregular rhythm notification feature on Apple Watch, can reduce the likelihood of stroke and improve health outcomes with earlier detection of atrial fibrillation
New Brunswick, NJ, February 25, 2020 — Johnson & Johnson (NYSE: JNJ) today announced that the Janssen Pharmaceutical Companies of Johnson & Johnson, in collaboration with Apple, opened enrollment for the Heartline Study. The study is designed to explore if the Heartline Study app on iPhone and heart health features on Apple Watch can improve health outcomes, including reducing the risk of stroke, with earlier detection of atrial fibrillation (AFib). AFib, a common form of irregular heart rhythm, is a leading cause of stroke in the U.S. To enroll in the Heartline Study, individuals must be age 65 or older, a U.S. resident, have Original (traditional) Medicare, own an iPhone 6s or a later model, and agree to provide access to their Medicare claims data. To learn more, determine eligibility to participate, and download the app, visit Heartline.com.
“Heartline is a study that has the potential to fundamentally change our understanding of how digital health tools, like the ECG app and irregular rhythm notification feature on Apple Watch, could lead to earlier detection of AFib, helping patients understand and directly engage in their heart health, prompting potentially life-saving conversations with their doctors, and improving health outcomes,” notes Dr. C. Michael Gibson*, Co-Chair of the Heartline Executive Committee and Professor of Medicine, Harvard Medical School and CEO, Baim Institute.
“The Heartline™ Study from Johnson & Johnson, in collaboration with Apple, has the potential to fundamentally change our understanding of how technology can help improve health outcomes,ˮ says Heartline Executive Committee Co-Chair @CMichaelGibson Tweet
Despite the fact that AFib is a leading cause of stroke, people often do not experience symptoms, making it difficult to diagnose. More than 33 million people worldwide and up to six million Americans live with AFib.1,2 Up to 30% don’t even know they have it until a serious cardiovascular event, such as a stroke, occurs.3 According to the U.S. Centers for Disease Control and Prevention, AFib, the most common sustained cardiac arrhythmia, results in 158,000 deaths and 454,000 hospitalizations each year.2
“As we look to tackle some of the greatest health care challenges, we must bring the best minds and capabilities to the table,” said Paul Burton, M.D., Ph.D., FACC, Vice President, Medical Affairs, Internal Medicine, Janssen Scientific Affairs, LLC, a Janssen Pharmaceutical Company of Johnson & Johnson. “Through this important collaboration with Apple, we are pioneering new models that we hope can break down some of the most common barriers to participation in clinical studies. Our work continues to develop and deliver solutions for those impacted by AFib in the areas of detection, treatment and care, through novel approaches, so that we can potentially improve their lives today and well into the future.”
One of the key objectives of this nationwide, randomized study is to assess if a heart health engagement program provided through the Heartline Study app on iPhone, in combination with the ECG app and the irregular rhythm notification feature on Apple Watch, can reduce the likelihood of stroke and improve health outcomes with the earlier detection of AFib.
- The engagement program, via the Heartline Study app from Johnson & Johnson, will provide ongoing education, tips, surveys and questionnaires across many topics related to overall heart health throughout the two-year active engagement period.
- The ECG app can classify an electrocardiogram as sinus rhythm or AFib.
- The irregular rhythm notification feature will provide notifications of irregular heart rhythms suggestive of AFib.
Through the app-based approach, the study will enable participants to engage in the study remotely, right from their iPhone and in some cases an Apple Watch, rather than travel to a clinical trial site. This approach to conducting a clinical trial, if successful, could potentially save time and cost.
“Apple technology is making a meaningful impact on scientific research through the powerful capabilities of iPhone and Apple Watch, all with privacy at the center of the participant experience,” said Myoung Cha, Apple's Head of Health Strategic Initiatives. “The Heartline Study will help further understanding of how our technology could both contribute to science and help improve health outcomes, including reducing the risk of stroke.”
About the Heartline Study
The Heartline Study is a nationwide, randomized, controlled, app-based, virtual research study sponsored by Janssen Pharmaceuticals, Inc., a member of the Janssen Pharmaceutical Companies of Johnson & Johnson. The team worked with Apple to jointly design the research study and the Heartline Study app. The study brings together Johnson & Johnson’s health expertise with Apple’s expertise in design, technology and privacy. Evidation Health, a collaborator in the study, provides the technology and study operations that enable the Heartline Study app and study experience for participants.
To be eligible for the study, participants must be age 65 or older, a resident of the United States for the duration of the study, have Original (traditional) Medicare, have an iPhone 6s or a later model (with iOS version 12.2 or later), and agree to provide access to their Medicare claims data. Additional entry criteria may apply. Eligible participants will be randomized to one of two possible groups. One group will participate by only using the Heartline Study app on their iPhone. The other group will participate by using the study app on their iPhone in addition to obtaining an Apple Watch to use the ECG app and irregular rhythm notification feature. Participants who already own an Apple Watch may be eligible to join the study as well, with certain restrictions.
Participation in the study will span a total of three years with two years of active engagement, followed by one year of additional data collection. During the active engagement period, participants will receive heart health education, wellness tips, surveys, and questionnaires across multiple topics related to overall heart health in the app each week.
About Johnson & Johnson
At Johnson & Johnson, we believe good health is the foundation of vibrant lives, thriving communities and forward progress. That's why for more than 130 years, we have aimed to keep people well at every age and every stage of life. Today, as the world's largest and most broadly-based health care company, we are committed to using our reach and size for good. We strive to improve access and affordability, create healthier communities, and put a healthy mind, body and environment within reach of everyone, everywhere. We are blending our heart, science and ingenuity to profoundly change the trajectory of health for humanity.
About the Janssen Pharmaceutical Companies of Johnson & Johnson
At Janssen, we’re creating a future where disease is a thing of the past. We’re the Pharmaceutical Companies of Johnson & Johnson, working tirelessly to make that future a reality for patients everywhere by fighting sickness with science, improving access with ingenuity, and healing hopelessness with heart. We focus on areas of medicine where we can make the biggest difference: Cardiovascular & Metabolism, Immunology, Infectious Diseases & Vaccines, Neuroscience, Oncology, and Pulmonary Hypertension. Follow us @JanssenGlobal. Janssen Pharmaceuticals, Inc. and Janssen Scientific Affairs, LLC are both part of the Janssen Pharmaceutical Companies of Johnson & Johnson.
Cautions Concerning Forward-Looking Statements
This press release contains "forward-looking statements" as defined in the Private Securities Litigation Reform Act of 1995 regarding the Heartline Study. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Janssen Pharmaceuticals, Inc., any of the other Janssen Pharmaceutical Companies and/or Johnson & Johnson. Risks and uncertainties include, but are not limited to: challenges and uncertainties inherent in product research and development, including the uncertainty of clinical success and of obtaining regulatory approvals; uncertainty of commercial success; competition, including technological advances, new products and patents attained by competitors; challenges to patents; changes in behavior and spending patterns of purchasers of health care products and services; changes to applicable laws and regulations, including global health care reforms; and trends toward health care cost containment. A further list and descriptions of these risks, uncertainties and other factors can be found in Johnson & Johnson's Annual Report on Form 10-K for the fiscal year ended December 29, 2019, including in the sections captioned “Cautionary Note Regarding Forward-Looking Statements” and “Item 1A. Risk Factors,” and in the company’s most recently filed Quarterly Report on Form 10-Q, and the company’s subsequent filings with the Securities and Exchange Commission. Copies of these filings are available online at www.sec.gov, www.jnj.com or on request from Johnson & Johnson. None of the Janssen Pharmaceutical Companies nor Johnson & Johnson undertakes to update any forward-looking statement as a result of new information or future events or developments.
MEDIA CONTACT:
Kristina Chang
+1 (201) 213-4115
[email protected]
INVESTOR RELATIONS CONTACT:
Lesley Fishman
+1 (732) 524-3922
[email protected]
1 Morillo CA, Banerjee A, Perel P, Wood D, Jouven X. Atrial fibrillation: the current epidemic. J Geriatr Cardiol. 2017;14(3):195–203. doi:10.11909/j.issn.1671-5411.2017.03.011
2 https://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_atrial_fibrillation.htm
3 Reiffel JA, Verma A, Kowey PR, et al. Incidence of Previously Undiagnosed Atrial Fibrillation Using Insertable Cardiac Monitors in a High-Risk Population: The REVEAL AF Study. JAMA Cardiol. 2017;2(10):1120–1127. doi:10.1001/jamacardio.2017.3180.
* Dr. Gibson is a paid consultant for Janssen. He has not been compensated for any media work.