FDA approves Novartis Piqray® – the first and only treatment specifically for patients with a PIK3CA mutation in HR+/HER2- advanced breast cancer
- Piqray (alpelisib, formerly BYL719) plus fulvestrant nearly doubled median PFS (11.0 vs 5.7 months) in HR+/HER2- advanced breast cancer patients with a PIK3CA mutation compared to fulvestrant alone in the SOLAR-1 clinical trial1,2,3,4
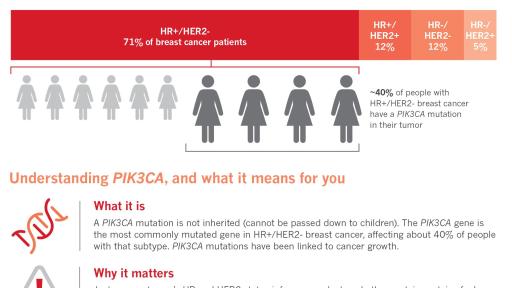
- ~40% of HR+/HER2- advanced breast cancer patients may face worse disease prognosis due to presence of PIK3CA mutations in their tumors5,6,7,8,9
- Piqray was the first new drug application approved under the FDA Oncology Center of Excellence Real-Time Oncology Review pilot program
East Hanover, N.J., May 24, 2019 – Novartis today announced the US Food and Drug Administration (FDA) has approved Piqray® (alpelisib, formerly BYL719) in combination with fulvestrant for the treatment of postmenopausal women, and men, with hormone receptor positive, human epidermal growth factor receptor-2 negative (HR+/HER2-), PIK3CA-mutated, advanced or metastatic breast cancer, as detected by an FDA-approved test following progression on or after an endocrine-based regimen1.
PIK3CA is the most commonly mutated gene in HR+/HER2- breast cancer; approximately 40% of patients living with HR+/HER2- breast cancer have this mutation8,10. PIK3CA mutations are associated with tumor growth, resistance to endocrine treatment and a poor overall prognosis.11,12. Piqray targets the effect of PIK3CA mutations and may help overcome endocrine resistance in HR+ advanced breast cancer.
“The FDA approval of Piqray, which was discovered at the Novartis Institutes for BioMedical Research, marks the first ever treatment specifically for HR+/HER2- advanced breast cancer with a PIK3CA mutation. We are proud to offer a new treatment option that specifically addresses the needs of the patients living with this mutation,” said Susanne Schaffert, PhD, CEO, Novartis Oncology. “We are grateful to our researchers’ bold and unrelenting pursuit of a first-in-class treatment for this incurable disease, and to the patients, investigators and administrators who participated in the clinical trials leading to this remarkable milestone.”
FDA approval is based on results of the Phase III trial, SOLAR-1, that showed Piqray plus fulvestrant nearly doubled median progression-free survival (PFS) compared to fulvestrant alone in HR+/HER2- advanced breast cancer patients with a PIK3CA mutation (median PFS 11.0 months vs 5.7 months; HR=0.65, 95% CI: 0.50-0.85; p<0.001)2. Piqray provided consistent PFS results across pre-specified subgroups, including among patients previously treated with a CDK4/6 inhibitor2,3.
Overall response rate (ORR), an indicator of the proportion of patients who experience at least a 30% reduction in overall tumor size (in patients with measurable disease), was more than doubled when Piqray was added to fulvestrant in patients with a PIK3CA mutation, (ORR= 35.7% vs 16.2% for fulvestrant alone, p=0.0002)2,3. Piqray and its associated companion diagnostic test from QIAGEN N.V. was the first combination product approved under the FDA Oncology Center of Excellence Real-Time Oncology Review pilot program.
“Today’s approval is expected to change the way we practice medicine in advanced breast cancer. For the first time, physicians can test for PIK3CA biomarkers and develop a treatment plan based on the genomic profile of a patient’s cancer,” said Fabrice André, MD, PhD, research director and head of INSERM Unit U981, professor in the Department of Medical Oncology at Institut Gustave Roussy in Villejuif, France, and global SOLAR-1 principal investigator. “In the SOLAR-1 Phase III trial, alpelisib plus fulvestrant nearly doubled median PFS and more than doubled overall response rate in patients with a PIK3CA mutation, offering them new hope for longer life without progression.”
Patients with HR+/HER2- advanced breast cancer can be selected for treatment with Piqray based on the presence of PIK3CA mutations. Concurrent with the approval of Piqray, the therascreen®* PIK3CA companion diagnostic test from QIAGEN was also approved by the FDA and is now available for patient testing.
“If you are facing a complex disease like metastatic breast cancer, you want to follow a path that is specific to your type of disease,” said Shirley Mertz, President, Metastatic Breast Cancer Network. “Finding the right treatment team and getting the right tests, like testing for the PIK3CA mutation, will help your healthcare team identify accurate, precise treatment options for your disease.”
Novartis is committed to providing patients with access to medicines, as well as resources and support to address a range of needs. The Novartis Oncology Patient Support Program is available to help guide eligible patients through the various aspects of getting started on treatment, from providing educational information to helping them understand their insurance coverage and identify potential financial assistance options. For more information, patients and healthcare professionals can call 1-800-282-7630.
Full prescribing information for Piqray can be found at https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/piqray.pdf.
About Piqray® (alpelisib)
Piqray is a kinase inhibitor approved in combination with fulvestrant for the treatment of postmenopausal women, and men, with HR+/HER2-, PIK3CA-mutated, advanced or metastatic breast cancer, as detected by an FDA-approved test following progression on or after endocrine-based regimen.1
Approximately 40% of HR+ advanced breast cancer patients have a mutation that may activate the PI3K-alpha isoform, called PIK3CA mutations5,6,7,8. These mutations are associated with resistance to endocrine therapy, disease progression and a poor prognosis11,12. Piqray works by inhibiting the PI3K pathway, predominantly the PI3K-alpha isoform, to address the effect of PIK3CA mutations.
About SOLAR-1
SOLAR-1 is a global, Phase III randomized, double-blind, placebo-controlled trial studying Piqray in combination with fulvestrant for postmenopausal women, and men, with PIK3CA-mutated HR+/HER2- advanced or metastatic breast cancer that progressed on or following aromatase inhibitor treatment with or without a CDK4/6 inhibitor1,2,3. SOLAR-1 is the pivotal Phase III trial that supported this approval.
The trial randomized 572 patients. Patients were allocated based on central tumor tissue assessment to either a PIK3CA-mutated cohort (n=341) or a PIK3CA non-mutated cohort (n=231). Within each cohort, patients were randomized in a 1:1 ratio to receive continuous oral treatment with Piqray (300 mg once daily) plus fulvestrant (500 mg every 28 days + Cycle 1 Day 15) or placebo plus fulvestrant. Stratification was based on visceral metastases and prior CDK4/6 inhibitor treatment1,2,3. Patients and investigators are blinded to PIK3CA mutation status and treatment.
The primary endpoint is local investigator assessed PFS using RECIST 1.1 for patients with a PIK3CA mutation. The key secondary endpoint is overall survival, and additional secondary endpoints include, but are not limited to, overall response rate, clinical benefit rate, health-related quality of life, efficacy in PIK3CA non-mutated cohort, safety and tolerability1,2,3. SOLAR-1 is ongoing to assess overall survival and other secondary endpoints.
About Novartis in Advanced Breast Cancer
For more than 30 years, Novartis has been tackling breast cancer with superior science, great collaboration and a passion for transforming patient care. With one of the most diverse breast cancer pipelines and one of the largest numbers of breast cancer compounds in development, Novartis leads the industry in discovery of new therapies and combinations, especially in HR+ advanced breast cancer, the most common form of the disease.
Indication
PIQRAY® (alpelisib) tablets is a prescription medicine used in combination with the medicine fulvestrant to treat women who have gone through menopause and men who have hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced breast cancer or breast cancer that has spread to other parts of the body (metastatic), with an abnormal phosphatidylinositol-3-kinase catalytic subunit alpha (PIK3CA) gene, and whose disease has progressed on or after endocrine therapy. Your health care provider will test your cancer for an abnormal “PIK3CA” gene to make sure that PIQRAY is right for you. It is not known if PIQRAY is safe and effective in children.
Important Safety Information
Patients should not take PIQRAY if they have had a severe allergic reaction to PIQRAY or are allergic to any of the ingredients in PIQRAY.
PIQRAY may cause serious side effects. PIQRAY can cause severe allergic reactions. Patients should tell their health care provider or get medical help right away if they have trouble breathing, flushing, rash, fever, or fast heart rate during treatment with PIQRAY. PIQRAY can cause severe skin reactions. Patients should tell their health care provider or get medical help right away if they get severe rash or rash that keeps getting worse, reddened skin, flu-like symptoms, blistering of the lips, eyes or mouth, blisters on the skin or skin peeling, with or without fever. PIQRAY can cause high blood sugar levels (hyperglycemia). Hyperglycemia is common with PIQRAY and can be severe. Health care providers will monitor patients’ blood sugar levels before they start and during treatment with PIQRAY. Health care providers may monitor patients’ blood sugar levels more often if they have a history of Type 2 diabetes. Patients should tell their health care provider right away if they develop symptoms of hyperglycemia, including excessive thirst, dry mouth, urinate more often than usual or have a higher amount of urine than normal, or increased appetite with weight loss. PIQRAY can cause lung problems (pneumonitis). Patients should tell their health care provider right away if they develop new or worsening symptoms of lung problems, including shortness of breath or trouble breathing, cough, or chest pain. Diarrhea is common with PIQRAY and can be severe. Severe diarrhea can lead to the loss of too much body water (dehydration) and kidney problems. Patients who develop diarrhea during treatment with PIQRAY should tell their health care provider right away.
Before taking PIQRAY, patients should tell their health care provider if they have a history of diabetes, skin rash, redness of skin, blistering of the lips, eyes or mouth, or skin peeling, are pregnant, or plan to become pregnant as PIQRAY can harm their unborn baby. Females who are able to become pregnant should use effective birth control during treatment with PIQRAY and for 1 week after the last dose. Do not breastfeed during treatment with PIQRAY and for 1 week after the last dose. Males with female partners who are able to become pregnant should use condoms and effective birth control during treatment with PIQRAY and for 1 week after the last dose. Patients should also read the Full Prescribing Information of fulvestrant for important pregnancy, contraception, infertility, and lactation information.
Patients should tell their health care provider all of the medicines they take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. PIQRAY and other medicines may affect each other causing side effects. Know the medicines you take. Keep a list of them to show your health care provider or pharmacist when you get a new medicine.
The most common side effects of PIQRAY when used with fulvestrant are rash, nausea, tiredness and weakness, decreased appetite, mouth sores, vomiting, weight loss, hair loss, and changes in certain blood tests.
Please see full Prescribing Information for Piqray, available at
https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/piqray.pdf.
Disclaimer
This press release contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements can generally be identified by words such as “potential,” “can,” “will,” “plan,” “expect,” “anticipate,” “look forward,” “believe,” “committed,” “investigational,” “pipeline,” “launch,” or similar terms, or by express or implied discussions regarding potential marketing approvals, new indications or labeling for the investigational or approved products described in this press release, or regarding potential future revenues from such products. You should not place undue reliance on these statements. Such forward-looking statements are based on our current beliefs and expectations regarding future events, and are subject to significant known and unknown risks and uncertainties. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those set forth in the forward-looking statements. There can be no guarantee that the investigational or approved products described in this press release will be submitted or approved for sale or for any additional indications or labeling in any market, or at any particular time. Nor can there be any guarantee that such products will be commercially successful in the future. In particular, our expectations regarding such products could be affected by, among other things, the uncertainties inherent in research and development, including clinical trial results and additional analysis of existing clinical data; regulatory actions or delays or government regulation generally; global trends toward health care cost containment, including government, payor and general public pricing and reimbursement pressures and requirements for increased pricing transparency; our ability to obtain or maintain proprietary intellectual property protection; the particular prescribing preferences of physicians and patients; general political and economic conditions; safety, quality or manufacturing issues; potential or actual data security and data privacy breaches, or disruptions of our information technology systems, and other risks and factors referred to in Novartis AG’s current Form 20-F on file with the US Securities and Exchange Commission. Novartis is providing the information in this press release as of this date and does not undertake any obligation to update any forward-looking statements contained in this press release as a result of new information, future events or otherwise.
About Novartis
Novartis is reimagining medicine to improve and extend people's lives. As a leading global medicines company, we use innovative science and digital technologies to create transformative treatments in areas of great medical need. In our quest to find new medicines, we consistently rank among the world's top companies investing in research and development. Novartis products reach more than 750 million people globally, and we are finding innovative ways to expand access to our latest treatments. About 105,000 people of more than 140 nationalities work at Novartis around the world. Novartis Pharmaceuticals Corporation, a US affiliate of Novartis, is located in East Hanover, NJ. Find out more at www.novartis.com.
Novartis is on Twitter. Sign up to follow @Novartis at http://twitter.com/novartis and @NovartisCancer at https://twitter.com/novartiscancer For Novartis multimedia content, please visit www.novartis.com/news/media-library For questions about the site or required registration, please contact [email protected]
References
- Piqray (alpelisib) Prescribing Information. East Hanover., New Jersey, USA: Novartis Pharmaceuticals Corporation; May 2019.
- André F, Ciruelos E, Rubovszky G. Alpelisib for PIK3CA-Mutated, Hormone-Receptor-Positive Advanced Breast Cancer. N Eng J Med 2019.
- André F, Ciruelos EM, Rubovszky G et al. Alpelisib (ALP) + fulvestrant (FUL) for advanced breast cancer (ABC): Results of the phase III SOLAR-1 trial. Annals of Oncology, Vol 29, Suppl 8, October 2018, Abstract LBA3_PR.
- Juric D, Ciruelos EM, Rubovszky G et al. Alpelisib (ALP) + fulvestrant (FUL) for advanced breast cancer (ABC): Phase 3 SOLAR-1 trial results. Presented at the San Antonio Breast Cancer Symposium (SABCS) (Abstract #GS3-08) on December 6, 2018.
- Tolaney S, Toi M, Neven P, et al. Presented at: 2019 American Association for Cancer Research (AACR) Annual Meeting; March 29-April 3, 2019; Atlanta, GA.
- Di Leo A, Johnston S, Seok Lee K, et al. Lancet Oncol. 2018;19(1):87-100.
- Moynahan ME, Chen D, He W, et al. Br J Cancer. 2017;116(6):726-730002E
- The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61-70.
- Sobhani N, Roviello G, Corona SP et al. The prognostic value of PI3K mutational status in breast cancer: a meta-analysis. J Cell Biochem. 2018;119(6):4287-4292.
- Sabine V, Crozier C, Brookes C, et al. Mutational analysis of PI3K/AKT signaling pathway in tamoxifen exemestane adjuvant multinational pathology study. Journal of Clinical Oncology. 2014;32:2951-2958.
- Miller TW, Rexer BN, Garrett JT, et al. Mutations in the Phosphatidylinositol 3-Kinase Pathway: Role in Tumor Progression and Therapeutic Implications in Breast Cancer. Breast Cancer Res. 2011.
- Saal LH, Johansson P, Holm K. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. PNAS. 2007;104(18):7564-7569.
*therascreen is a registered trademark of QIAGEN N.V.
Novartis Media Relations
E-mail: [email protected]
Julie Masow
Novartis Oncology Media Relations
+1 862 778 7220 (direct)
+1 862 579 8456 (mobile)
[email protected]
Eric Althoff
Novartis US External Communications
+1 646 438 4335
[email protected]
Novartis Investor Relations
Central investor relations line: +41 61 324 7944
E-mail: [email protected]
| Central | North America | ||
| Samir Shah | +41 61 324 7944 | Richard Pulik | +1 862 778 3275 |
| Pierre-Michel Bringer | +41 61 324 1065 | Cory Twining | +1 862 778 3258 |
| Thomas Hungerbuehler | +41 61 324 8425 | ||
| Isabella Zinck | +41 61 324 7188 |
The FDA approved a new treatment for HR+/HER2- MBC patients with a mutation Tweet