SENTARA HEALTHCARE CLINICAL TRIAL FINDS COPPER-INFUSED PRODUCTS SIGNIFICANTLY REDUCE HOSPITAL-ACQUIRED INFECTIONS
Sentara Leigh Hospital trial published in peer-reviewed journal, presented at national conference
OVERVIEW
- Sentara conducted the world’s largest clinical trial of copper against hospital-acquired infections (HAIs).
- Copper-infused linens produced by Richmond-based Cupron, Inc. and hard surfaces developed and manufactured by Norfolk-based EOS Surfaces, LLC were deployed in 124 patient rooms in a new patient tower while a 70s-era wing served as a control.
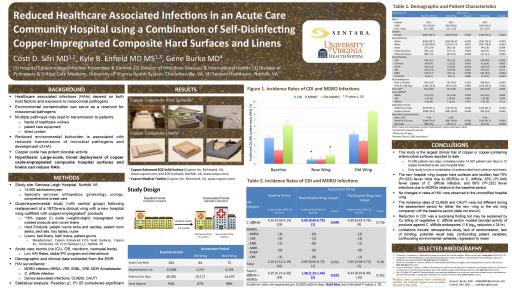
- Copper-infused hard surfaces and linens were found to contribute to an 83% reduction in C-difficile alone and a 78% overall reduction in a host of multi-drug resistant organisms (MDROs) including C-diff, MRSA and VRE.
- Results were published in the American Journal of Infection Control on Sept. 28 and will be presented at the annual ID Week conference of the Infectious Disease Society of America on Oct. 27, 2016.
PR Newswire, NORFOLK, VA - October 26, 2016
A ten-month clinical trial at Sentara Leigh Hospital in Norfolk, Virginia has determined that hard surfaces and linens infused with copper oxide compounds contributed to an 83% reduction in C-difficile and a 78% overall reduction in a host of multi-drug resistant organisms (MDROs) including C-diff, MRSA and VRE in a real-world clinical environment. These results occurred in a hospital with a robust protocol for managing infection risk certified by the health care accrediting body DNV-GL Healthcare.
The results of the trial were published in the peer-reviewed American Journal of Infection Control on Sept. 28, 2016 and will be presented at the annual conference of the Infectious Disease Society of America in New Orleans, Louisiana on October 27, 2016.
“We’ve just about reached the limit of human processes to control infections in hospitals and Sentara Leigh was already performing pretty well,” said Gene Burke, MD, vice president and executive medical director for clinical effectiveness with Sentara Healthcare. “We owe it to our patients to try new approaches.” Sentara is an integrated not-for-profit system with 12 hospitals in Virginia and North Carolina.
“The clinical trial just published reflects the Sentara commitment to innovation,” says Howard P. Kern, president and CEO of Sentara Healthcare. “We are relentless in the pursuit of improved clinical outcomes and an exceptional patient experience and these copper products are helping us achieve both of those goals.”
“These results suggest that antimicrobial surfaces and linens may have substantial impact in reducing HAIs due to problematic MDROs in a hospital that has already employed aggressive infection control measures and has low rates of HAIs,” according to the study paper, which was co-authored by Costi Sifri, MD, associate professor of medicine and director of hospital epidemiology at the University of Virginia Health System and Kyle Enfield, MD, associate professor of medicine at the UVA School of Medicine, plus Dr. Burke of Sentara.
Three companies engaged in clinical trial
Sentara Healthcare partnered with Richmond, Virginia-based Cupron, Inc., which invented the proprietary copper oxide technology used in the surfaces and textiles, and Norfolk, Virginia-based EOS Surfaces, LLC, which developed the unique copper oxide-impregnated hard surfaces. EOS Surfaces manufactured custom-made countertops, bathroom sinks, bedside tables and bedrails that were installed in 124 patient rooms in the newly-built East Tower at Sentara Leigh, plus copper-infused work surfaces at nursing and charting stations, in soil rooms and other common areas.
EOScu, produced by EOS Surfaces, LLC, is trademarked as a Preventive|Biocidal Surfaces™ and the only synthetic hard surface registered by the Environmental Protection Agency as a bacterial sanitizer proven to kill 99.9% of bacteria within two hours of contact. The EPA granted federal registration only after EOS submitted 14,000 production samples for rigorous testing and validation of efficacy claims.
“EPA registration gives us credibility and opens the door to real conversations with people looking to improve their clinical performance,” says Ken Trinder, CEO of EOS Surfaces, LLC.
Cupron, Inc. produces Cupron Medical Textiles including bedsheets, pillow cases, thermal blankets and patient gowns, plus towels, wash cloths and bath blankets used in patients’ private bathrooms.
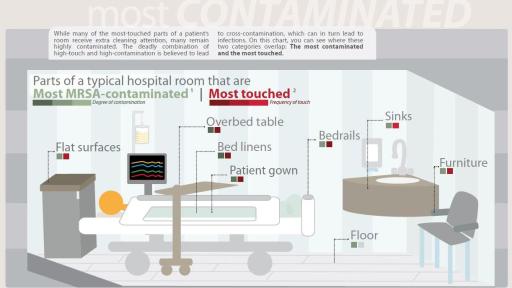
“Studies show that patient gowns and bed linens are the two most contaminated surfaces in a room,” says Chris Andrews, CEO of Cupron, Inc. “With our sheets and gowns, all the soft surfaces with which the patient has intimate contact are actively killing bacteria.”
Copper products require no new processes, staff training or special maintenance, Dr. Burke adds. “We sanitize patient rooms every day, but that leaves 23-and-a-half hours for bacteria to proliferate, but copper keeps killing around the clock.”
Sentara is committed to copper
Sentara Healthcare is confident enough in copper that copper-infused bedside tables and bed rails will be retro-fitted in all 12 Sentara hospitals and copper-infused linens will be deployed in all patient rooms.
About Sentara Healthcare - Sentara Healthcare, based in Norfolk, VA, is an integrated not-for-profit system of 12 hospitals in Virginia and North Carolina strategically focused on continuous improvement in quality, safety, and the patient experience.
About Cupron - Cupron, Inc., is a global healthcare sciences company focused upon healthcare and healthy lifestyle applications. Based in Richmond, Va., with an office in Herzliya, Israel, Cupron has patented methods for delivering the unique antimicrobial and health properties of copper to a range of textile, surfaces and other polymeric materials products.
About EOS Surfaces - EOS Surfaces, LLC, an innovative surfacing technology company, conceptualized and created EOSCU, the industry’s only practical hard surface that is EPA-registered, continuously killing harmful bacteria*. The company also developed Health. Care. | An Educational Blog that serves as a resource platform for patients, physicians and the hospital C-suite.
*Testing demonstrates effective antibacterial activity against Staphylococcus aureus, Enterobacter aerogenes, Methicillin-Resistant Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa.
Media Contacts
Sentara Healthcare Dale Gauding 757-615-4718 [email protected]
Cupron, Inc. Jason Ellis 804-306-1370 [email protected]
EOS Surfaces, LLC Tanya Kaish Keller 757-618-3655 [email protected]
University of Virginia Eric Swensen 434-924-5770 [email protected]
24-HOUR MEDIA PAGER: 757.671.4971
New clinical trial by @SentaraHealth finds #copper reduces #HAIs @Cupron @EOSSurfaces #idweek2016 Tweet