Sanofi Pasteur Ships First of its 2016-2017 Seasonal Influenza Vaccine Doses in United States
- World’s largest influenza vaccine manufacturer expects to distribute more than 65 million vaccine doses to help protect people six months and older against the flu -
- Shipments to health care providers and distributors mark the start of influenza immunization season -
- Approximately 60 percent of vaccinated seniors expected to receive Fluzone High-Dose vaccine this season -
PR Newswire, SWIFTWATER, Pa. – July 26, 2016
Sanofi Pasteur, the vaccines division of Sanofi (EURONEXT: SAN and NYSE: SNY), announced today that its first doses of Fluzone® (Influenza Vaccine) for the 2016-2017 influenza (“flu”) season have been released by the U.S. Food and Drug Administration (FDA) for shipment. This represents the first of more than 65 million total doses of seasonal influenza vaccine manufactured by Sanofi Pasteur that will be delivered to U.S. health care providers and pharmacies beginning in July and continuing throughout the remainder of the year. Sanofi Pasteur plans to increase its supply to respond to the shifting pediatric public health needs.
Seasonal influenza activity typically occurs between October and May and peaks between December and February. However, influenza activity peaked noticeably late last season occurring in early March 2016.1 Influenza seasons are always unpredictable as new influenza strains emerge and strain activity fluctuates throughout the year, making timely vaccination even more important to help protect against the virus, especially for seniors, young children and infants six months of age and older.
The Centers for Disease Control and Prevention (CDC) recommends annual influenza vaccination for everyone six months of age and older, with rare exception, and recommends receiving the vaccine as soon as it is available to help with prevention even before the season begins. In the 2015-2016 influenza season, 50 percent of seniors who were vaccinated received Fluzone High-Dose vaccine, and this number is expected to rise to 60 percent in the coming season.
“There is general awareness of influenza among the public given its widespread prevalence,” said David P. Greenberg, M.D., Associate Vice President and Regional Medical Head North America, Sanofi Pasteur. “What is not well known is that influenza can be life-threatening and have a lasting health impact, especially for the most vulnerable populations. In the 2014-2015 season, the CDC estimated 40 million flu illnesses, 19 million flu-associated medical visits and almost 1 million flu-associated hospitalizations.”2
As the single largest provider of influenza vaccine to the United States, Sanofi Pasteur provides a wide range of influenza vaccine options. The Fluzone vaccine portfolio meets immunization needs across the entire lifespan, from children as young as six months of age through adults 65 years of age and older, and includes the following products:
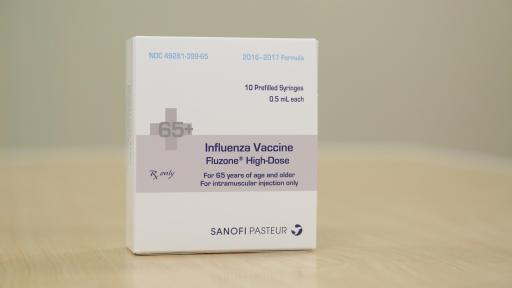
- Fluzone High-Dose vaccine, the first influenza vaccine designed specifically for people 65 years of age and older, is an inactivated influenza vaccine.3 Fluzone High-Dose vaccine is the only influenza vaccine for seniors that the U.S. Food and Drug Administration (FDA) has approved as having demonstrated superior efficacy against influenza compared with Fluzone vaccine. It contains four times the antigen content of Fluzone vaccine to help promote a stronger immune response to influenza.4 Clinical data published in The New England Journal of Medicine demonstrated that Fluzone High-Dose vaccine was 24.2 percent more effective than Fluzone vaccine in preventing laboratory-confirmed influenza caused by any influenza viral type or subtype in association with influenza-like illness, in adults 65 years of age and older.4 A secondary endpoint demonstrated Fluzone High-Dose vaccine was 51.1 percent more effective than Fluzone vaccine against influenza caused by viral strains antigenically similar to those contained in the vaccine using a modified CDC influenza-like illness definition.4
- Fluzone Quadrivalent vaccine helps protect against four influenza strains (two A strains and two B strains). The influenza B strain is associated with substantial hospitalization and mortality, especially in children and young adults. In fact, on average, over multiple recent seasons, 34 percent of influenza-related deaths in children up to 18 years of age were due to influenza B.5 Fluzone Quadrivalent vaccine is the first four-strain influenza vaccine licensed for use in people six months of age and older.
- Fluzone Intradermal Quadrivalent vaccine was licensed by the U.S. Food and Drug Administration (FDA) in 2014 for adults 18 through 64 years of age. Fluzone Intradermal Quadrivalent vaccine is administered directly into the skin using a 90 percent smaller, 1.5 mm microneedle and offers four-strain protection that is convenient, efficient, and easy to use, allowing for streamlined administration by health care providers.
“Sanofi Pasteur is at the forefront of influenza vaccine innovation,” said Dr. Greenberg. “Our legacy for researching and manufacturing influenza vaccines has existed for nearly 70 years at our Swiftwater-based facility, demonstrating our experience and commitment to this important public health need. We are dedicated to continuously improving our influenza vaccine options to ultimately help protect against influenza strains, including drifted strains that may occur in the future.”
To address recommendations by the Advisory Committee on Immunization Practices (ACIP), Sanofi Pasteur has increased production of Fluzone Quadrivalent vaccine where possible. Sanofi Pasteur is the sole provider of injectable influenza vaccine for pediatric patients six months through 35 months of age.
Health care providers who placed reservations with Sanofi Pasteur should expect to receive initial shipments between late July and early August to support fall immunization campaigns. Health care providers wishing to reserve vaccine can do so by visiting www.vaccineshoppe.com or by calling 1-800-VACCINE (1-800-822-2463). Members of the public seeking a specific vaccine option, such as Fluzone High-Dose vaccine, Fluzone Intradermal Quadrivalent vaccine, or Fluzone Quadrivalent vaccine, can search for local providers at www.Fluzone.com.
About Sanofi Pasteur Influenza Vaccine Manufacturing
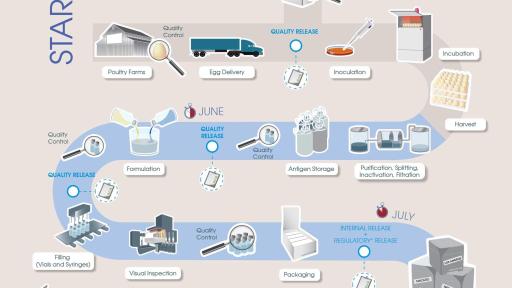
As the world leader in the research, development and manufacturing of seasonal influenza vaccines, Sanofi Pasteur uses the most innovative technology and processes. For more information about Sanofi Pasteur’s influenza vaccine manufacturing process, please view the following infographic (“Production Process for the United States Infographic”) and watch a video that provides a glimpse into the Sanofi Pasteur production facilities.
About Influenza
Influenza is a serious respiratory illness that is easily spread and can lead to severe complications, even death. Each year, one out of every five Americans gets the flu and, on average, more than 200,000 people are hospitalized from influenza-related complications.6 Influenza seasons are unpredictable with flu-related deaths ranging from 3000 to as high as 49,000 people.7 Combined with pneumonia, influenza is one of the top ten leading causes of death in the United States.8 Vaccination is safe and effective and the best way to help prevent influenza and its complications.
Children six months through eight years of age may require two doses of vaccine for the 2016-2017 influenza season. Parents should consult their health care provider about the number of doses of influenza vaccine their child should receive.
Individuals who are not immunized early in the season will still have time do so as the influenza vaccination is beneficial throughout the season, even into the spring, as long as influenza viruses are still in circulation.9
About Fluzone Vaccines
Indication
Fluzone Quadrivalent, Fluzone Intradermal Quadrivalent, and Fluzone High-Dose vaccines are given to help prevent influenza disease caused by influenza A and B strains contained in each vaccine.
Fluzone Quadrivalent vaccine is given to people 6 months of age and older. Fluzone Intradermal Quadrivalent vaccine is given to people 18 through 64 years of age. Fluzone High-Dose vaccine is given to people 65 years of age and older.
Safety Information
Side effects to Fluzone Quadrivalent, Fluzone Intradermal Quadrivalent, and Fluzone High-Dose vaccines include pain and swelling at the injection site (also itching in adults receiving Fluzone Intradermal Quadrivalent vaccine); muscle aches, fatigue, headache, and fever (also irritability, abnormal crying, drowsiness, appetite loss, and vomiting in young children receiving Fluzone Quadrivalent vaccine). Itching, redness, swelling, and firmness at the injection site occurred more frequently with Fluzone Intradermal vaccine (containing 3 influenza strains) than with Fluzone vaccine. Other side effects may occur.
Fluzone Quadrivalent, Fluzone Intradermal Quadrivalent, and Fluzone High-Dose, vaccines should not be administered to anyone with a severe allergic reaction (e.g., anaphylaxis) to any vaccine component, including eggs, egg products, or thimerosal (the multidose vial is the only presentation containing thimerosal), or to a previous dose of any influenza vaccine.
Tell the doctor if you/your child has ever experienced Guillain-Barré syndrome (severe muscle weakness) after a previous dose of influenza vaccine. If you notice any other problems or symptoms following vaccination, please contact your health care professional immediately. Vaccination with Fluzone Quadrivalent, Fluzone Intradermal Quadrivalent, or Fluzone High-Dose vaccine may not protect all individuals.
For more information about Fluzone Quadrivalent, Fluzone Intradermal Quadrivalent, or Fluzone High-Dose vaccine, talk to your health care professional and see complete Patient Information.
About Sanofi
Sanofi, a global healthcare leader, discovers, develops and distributes therapeutic solutions focused on patients' needs. Sanofi has core strengths in diabetes solutions, human vaccines, innovative drugs, consumer healthcare, emerging markets, animal health and Genzyme. Sanofi is listed in Paris (EURONEXT: SAN) and in New York (NYSE: SNY).
Sanofi Pasteur, the vaccines division of Sanofi, provides more than 1 billion doses of vaccine each year, making it possible to immunize more than 500 million people across the globe. A world leader in the vaccine industry, Sanofi Pasteur produces a portfolio of high quality vaccines that matches its areas of expertise and meets public-health demand. The company's heritage, to create vaccines that protect life, dates back more than a century. Sanofi Pasteur is the largest company entirely dedicated to vaccines. Every day, the company invests more than EUR 1 million in research and development. For more information, please visit: www.sanofipasteur.com or www.sanofipasteur.us
Forward Looking Statements
This press release contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. These statements include projections and estimates and their underlying assumptions, statements regarding plans, objectives, intentions and expectations with respect to future financial results, events, operations, services, product development and potential, and statements regarding future performance. Forward-looking statements are generally identified by the words “expects”, “anticipates”, “believes”, “intends”, “estimates”, “plans” and similar expressions. Although Sanofi’s management believes that the expectations reflected in such forward-looking statements are reasonable, investors are cautioned that forward-looking information and statements are subject to various risks and uncertainties, many of which are difficult to predict and generally beyond the control of Sanofi, that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include among other things, the uncertainties inherent in research and development, future clinical data and analysis, including post marketing, decisions by regulatory authorities, such as the FDA or the EMA, regarding whether and when to approve any drug, device or biological application that may be filed for any such product candidates as well as their decisions regarding labelling and other matters that could affect the availability or commercial potential of such product candidates, the absence of guarantee that the product candidates if approved will be commercially successful, the future approval and commercial success of therapeutic alternatives, the Group’s ability to benefit from external growth opportunities, trends in exchange rates and prevailing interest rates, the impact of cost containment initiatives and subsequent changes thereto, the average number of shares outstanding as well as those discussed or identified in the public filings with the SEC and the AMF made by Sanofi, including those listed under “Risk Factors” and “Cautionary Statement Regarding Forward-Looking Statements” in Sanofi’s annual report on Form 20-F for the year ended December 31, 2015. Other than as required by applicable law, Sanofi does not undertake any obligation to update or revise any forward-looking information or statements.
Contacts:
Global Media Relations
Alain Bernal
Tel. +33 (0)4 37 37 50 38
[email protected]
www.sanofipasteur.com
U.S./Country Media Relations
Cristine Schroeder
Tel. +1-570-957-3027
[email protected]
www.sanofipasteur.us
Investor Relations
George Grofik
Tel. +33 (0)1 53 77 45 45
[email protected]
1 Centers for Disease Control and Prevention (CDC). What You Should Know for the 2015-2016 Influenza Season. http://www.cdc.gov/flu/about/season/flu-season-2015-2016.htm. Accessed May 9, 2016.
2 CDC. What You Should Know for the 2014-2015 Influenza Season. http://www.cdc.gov/flu/pastseasons/1415season.htm. Accessed May 12, 2016.
3 Fluzone High-Dose vaccine [Prescribing Information]. Swiftwater, PA: Sanofi Pasteur Inc.; 2015.
4 DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371:635-645.
5 Ambrose CS, Levin MJ. The rationale for quadrivalent influenza vaccine. Hum Vaccin Immunother. 2012; 8:81-88
6 CDC. Seasonal Influenza-Associated Hospitalizations in the United States. http://www.cdc.gov/flu/about/qa/hospital.htm. Accessed May 12, 2016.
7 CDC. Estimating Seasonal Influenza-Associated Deaths in the United States: CDC Study Confirms Variability of Flu. http://www.cdc.gov/flu/about/disease/us_flu-related_deaths.htm. Accessed May 12, 2016.
8 Bastian AB, Kochanek DK, Murphy SL, et al. National Vital Statistics Reports. Pg 1. http://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_02.pdf. Accessed May 12, 2016.
9 CDC. Key Facts About Seasonal Flu Vaccine. http://www.cdc.gov/flu/protect/keyfacts.htm. Accessed May 12, 2016.