GSK ANNOUNCES NUCALA® (mepolizumab), NOW AVAILABLE IN THE US FOR SUB-GROUP OF PATIENTS WITH SEVERE ASTHMA
RESEARCH TRIANGLE PARK, N.C., Dec. 2, 2015 /PRNewswire/--GlaxoSmithKline plc (LSE/NYSE: GSK) today announced that Nucala® (mepolizumab), the first and only biologic add-on therapy for people 12 years and older with severe asthma with an eosinophilic phenotype, is now available by prescription in the US. Nucala is not indicated for the treatment of other eosinophilic conditions or relief of acute bronchospasm or status asthmaticus.
Nucala, administered by a healthcare professional as a 100mg fixed dose subcutaneous injection every 28 days, is offered to healthcare providers through a network of GSK-authorized wholesalers, specialty distributors, and specialty pharmacies.
To support the needs of appropriate patients with severe asthma who are prescribed Nucala, GSK also announced the creation of a complimentary patient support hotline, Gateway to Nucala, designed to provide personalized assistance. This program can be accessed at 844-4-NUCALA (844-468-2252). Program representatives are available from 8 AM to 8 PM ET, Monday through Friday.
“The goal of Gateway to Nucala is to support patients for who Nucala is appropriate with just one phone number where they can call and get answers to their questions about insurance coverage and access to treatment, and help alleviate any uncertainty in that regard. I encourage patients to call and enroll in the program,” said Anjana Narain, Vice President, Respiratory Biologics Specialty, GSK.
Gateway to Nucala supports patients and healthcare providers with live support on several topics, including help with understanding and navigating their insurance coverage for Nucala, and providing programs for eligible patients who may need help affording their drug.
For full US prescribing information, please click here.
About asthma
Current estimates indicate that as many as 242 million people live with asthma worldwide. It is estimated that in the US asthma affects 25.7 million individuals. For many of these patients, existing therapies can provide adequate control of their symptoms if used appropriately. However approximately 5% of patients with asthma cannot achieve symptom control with existing therapies.
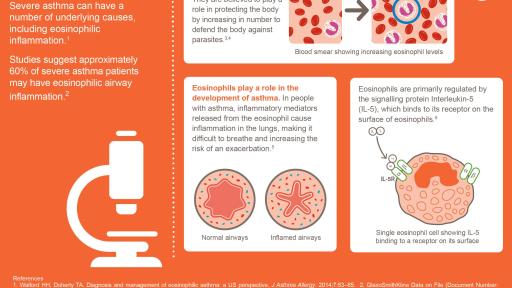
About severe asthma and eosinophil inflammation
Severe asthma is defined as “asthma which requires treatment with high dose inhaled corticosteroids
(ICS) plus a second controller (and/or systemic corticosteroids) to prevent it from becoming
‘uncontrolled’ or which remains ‘uncontrolled’ despite this therapy”. Severe asthma patients are also often characterized by long-term use of oral corticosteroids (OCS). In a sub-set of severe asthma patients, the over-production of eosinophils (a type of white blood cell) is known to cause
inflammation in the lungs that can affect the airways, limiting breathing and increasing the frequency
of asthma attacks. Interleukin-5 (IL-5) is the main promoter of eosinophil growth, activation and
survival and provides an essential signal for the movement of eosinophils from the bone marrow into
the lung. Studies suggest that approximately 60% of patients with severe asthma have eosinophilic
airway inflammation.
Important Safety Information (ISI) for Nucala
The following ISI is based on the Highlights section of the US Prescribing Information for Nucala.
Please consult the full Prescribing Information for all the labelled safety information for Nucala.
Nucala should not be administered to patients with a history of hypersensitivity to mepolizumab or excipients in the formulation.
Hypersensitivity reactions (e.g., angioedema, bronchospasm, hypotension, urticaria, rash) have occurred following administration of Nucala. These reactions generally occur within hours of administration, but in some instances can have a delayed onset (i.e., days). In the event of a hypersensitivity reaction, Nucala should be discontinued.
Do not use Nucala to treat acute bronchospasm or status asthmaticus. Patients should seek medical advice if their asthma remains uncontrolled or worsens after initiation of treatment with Nucala. In controlled clinical trials, two serious adverse reactions of herpes zoster occurred in subjects treated with Nucala compared with none in placebo. Consider varicella vaccination if medically appropriate prior to starting therapy with Nucala.
Do not discontinue systemic or inhaled corticosteroids abruptly upon initiation of therapy with Nucala. Reductions in corticosteroid dose, if appropriate, should be gradual and performed under the direct supervision of a physician.
Patients with known parasitic infections were excluded from participation in clinical trials. It is unknown if Nucala will influence a patient’s response against parasitic infections. Treat patients with pre-existing helminth infections before initiating therapy with Nucala. If patients become infected while receiving treatment with Nucala and do not respond to anti-helminth treatment, discontinue treatment with Nucala until infection resolves.
The most common adverse reactions (≥3% and more common than placebo) reported in the first 24 weeks of two clinical trials with Nucala (and placebo) were headache, 19% (18%); injection site reaction, 8% (3%); back pain, 5% (4%); fatigue, 5% (4%); influenza, 3% (2%); urinary tract infection 3% (2%); upper abdominal pain, 3% (2%); pruritis, 3% (2%); eczema, 3% (‹1%); and muscle spasm, 3% (‹1%).
In three Phase III trials, 10% of subjects in the Nucala group experienced systemic (allergic and non-allergic) reactions compared to 7% in the placebo group. Systemic allergic/hypersensitivity reactions were reported by 1% of subjects who received Nucala compared to 2% of subjects in the placebo group. Manifestations included rash, pruritus, headache, and myalgia. Systemic non-allergic reactions were reported by 2% of subjects in the Nucala group and 3% of subjects in the placebo group. Manifestations included rash, flushing, and myalgia. A majority of the systemic reactions were experienced on the day of dosing.
Injection site reactions (e.g., pain, erythema, swelling, itching, and burning sensation) occurred at a rate of 8% in subjects treated with Nucala compared with 3% in subjects treated with placebo. Nucala® is a registered trade mark of the GSK group of companies.
GSK – one of the world’s leading research-based pharmaceutical and healthcare companies – is committed to improving the quality of human life by enabling people to do more, feel better and live longer. For further information please visit www.gsk.com.
| UK Media enquiries: | David Mawdsley | +44 (0) 20 8047 5502 | (London) |
|---|---|---|---|
| Simon Steel | +44 (0) 20 8047 5502 | (London) | |
| David Daley | +44 (0) 20 8047 5502 | (London) | |
| Catherine Hartley | +44 (0) 20 8047 5502 | (London) | |
| Claire Brough | +44 (0) 20 8047 5502 | (London) | |
| US Media enquiries: | Sarah Alspach | +1 202 715 1048 | (Washington, DC) |
| Sarah Spencer | +1 215 751 3335 | (Philadelphia) | |
| Mary Anne Rhyne | +1 919 483 0492 | (North Carolina) | |
| Jenni Ligday | +1 202 715 1049 | (Washington, DC) | |
| Robin Gaitens | + 1 (919) 271 6560 | (North Carolina) | |
| Gwynn Oosterbaan | +1 215 751 7468 | (Philadelphia) | |
| Analyst/Investor enquiries: | Ziba Shamsi | +44 (0) 20 8047 5543 | (London) |
| Tom Curry | + 1 215 751 5419 | (Philadelphia) | |
| Gary Davies | +44 (0) 20 8047 5503 | (London) | |
| James Dodwell | +44 (0) 20 8047 2406 | (London) | |
| Jeff McLaughlin | +1 215 751 7002 | (Philadelphia) |
Cautionary statement regarding forward-looking statements
GSK cautions investors that any forward-looking statements or projections made by GSK, including those made in this announcement, are subject to risks and uncertainties that may cause actual results to differ materially from those projected. Such factors include, but are not limited to, those described under Item 3.D 'Risk factors' in the company's Annual Report on Form 20-F for 2014.
Registered in England & Wales: No. 3888792
Registered Office: 980 Great West Road Brentford, Middlesex TW8 9GS