Related Links
Please See Full Prescribing Information www.astrazeneca-us.comVideo Gallery
Photo Gallery
Download Video
Download Patient Video Download B-roll Download AstraZeneca SpokespeopleRelated Documents
Press Release Document 2TAGRISSO™ (OSIMERTINIB) (AZD9291) APPROVED BY THE US FDA AS TREATMENT FOR PATIENTS WITH EGFR T790M MUTATION-POSITIVE METASTATIC NON-SMALL CELL LUNG CANCER
One of fastest development programs – from start of clinical trials to approval in just over two and a half years to meet unmet patient need
With objective response rate of 59% and duration of response of 12.4 months, TAGRISSO provides important new option for patients
PR Newswire, (WILMINGTON, Del., November 13, 2015)
– AstraZeneca today announced that the US Food and Drug Administration (FDA) has approved TAGRISSO™ (osimertinib) (AZD9291) 80mg once-daily tablets for the treatment of patients with metastatic epidermal growth factor receptor (EGFR) T790M mutation-positive non-small cell lung cancer (NSCLC), as detected by an FDA-approved test, who have progressed on or after EGFR tyrosine kinase inhibitor (TKI) therapy.
TAGRISSO is the only approved medicine indicated for patients with metastatic EGFR T790M mutation-positive non-small cell lung cancer. This indication is approved under the FDA’s accelerated approval process based on tumor response rate and duration of response (DoR).
TAGRISSO is an EGFR-TKI, a targeted cancer therapy, designed to inhibit both the activating, sensitizing mutations (EGFRm), and T790M, a genetic mutation responsible to EGFR-TKI treatment resistance. Nearly two-thirds of NSCLC patients who are EGFR mutation-positive and experience disease progression after being treated with an EGFR-TKI develop the T790M resistance mutation, for which there have been limited treatment options.
Pascal Soriot, Chief Executive Officer, AstraZeneca, said: “The FDA approval of TAGRISSO marks an important milestone for lung cancer patients who urgently need new treatment options. We have built on our heritage in this area and acted on the breakthrough clinical evidence to ensure this next-generation medicine reaches patients in record time. As we advance our comprehensive lung cancer portfolio, we have the opportunity to treat greater numbers of patients across all stages of this disease through precision medicines, immunotherapies and novel combinations.”
Pasi A Jänne M.D., PhD, Director, Lowe Center for Thoracic Oncology at Dana-Farber Cancer Institute, Scientific Director, Belfer Center for Applied Cancer Science and Professor of Medicine, Harvard Medical School said: “In the AURA clinical studies, TAGRISSO has demonstrated compelling early efficacy and tolerability in patients with EGFRm T790M metastatic non-small cell lung cancer. This treatment has the potential to become the standard of care for patients living with EGFRm T790M non-small cell lung cancer. The accelerated approval of TAGRISSO highlights its clinical promise for a targeted group of patients and gives healthcare providers an important new option.”
AstraZeneca has collaborated with Roche to develop the cobas® EGFR Mutation Test v2 as the companion diagnostic for TAGRISSO. The cobas® EGFR Mutation Test v2 is intended to identify a range of EGFR mutations in patients with non-small cell lung cancer, including T790M.
TAGRISSO was granted Fast Track, Breakthrough Therapy, Priority Review and Accelerated Approval status by the FDA. In Europe and Japan, TAGRISSO was granted Accelerated Assessment and Priority Review status respectively. Interactions with regulatory authorities in the rest of the world are ongoing.
The FDA approval of TAGRISSO is based on data from the two AURA Phase II studies (AURA extension and AURA2) which demonstrated efficacy in 411 EGFRm T790M NSCLC patients that had progressed on or after an EGFR TKI. In those trials, overall objective response rate ((ORR) a measurement of tumor shrinkage) was 59% (95% CI: 54% to 64%). In a separate part of the AURA Study in 63 patients, ORR was 51% and median duration of response was 12.4 months.
The TAGRISSO tolerability profile showed that no individual severe grade 3+ adverse events occurred at ≥ 3.5%.The most common adverse events were generally mild to moderate and included diarrhea (42% all grades; 1.0% Grade 3/4), rash (41% all grades; 0.5% Grade 3/4), dry skin (31% all grades; 0% Grade 3/4), and nail toxicity (25% all grades; 0% Grade 3/4).
Osimertinib (AZD9291) Development Program
Osimertinib (AZD9291) is being studied in the confirmatory trial, AURA3, an open label, randomized Phase III study designed to assess the efficacy and safety of osimertinib (AZD9291) versus platinum-based doublet chemotherapy in patients with EGFR T790M positive, locally advanced, or metastatic NSCLC who have progressed following prior therapy with an EGFR-TKI. Osimertinib (AZD9291) is also being investigated in the adjuvant setting and in the metastatic first-line setting, including in patients with brain metastases, as well as in combination with other compounds.
Important Safety Information
- There are no contraindications for TAGRISSO
- Interstitial Lung Disease (ILD)/Pneumonitis occurred in 3.3% and were fatal in 0.5% of 813 TAGRISSO patients. Withhold TAGRISSO and promptly investigate for ILD in any patient presenting with worsening of respiratory symptoms indicative of ILD (e.g., dyspnea, cough and fever). Permanently discontinue TAGRISSO if ILD is confirmed
- QTc interval prolongation occurred in TAGRISSO patients. Of the 411 patients in two Phase II studies, one patient (0.2%) was found to have a QTc greater than 500 msec, and 11 patients (2.7%) had an increase from baseline QTc greater than 60 msec. Conduct periodic monitoring with ECGs and electrolytes in patients with congenital long QTc syndrome, congestive heart failure, electrolyte abnormalities, or those who are taking medications known to prolong the QTc interval. Permanently discontinue TAGRISSO in patients who develop QTc interval prolongation with signs/symptoms of life threatening arrhythmia
- Cardiomyopathy occurred in 1.4% and were fatal in 0.2% of 813 TAGRISSO patients. Left Ventricular Ejection Fraction (LVEF) decline >10% and a drop to <50% occurred in 2.4% of (9/375) TAGRISSO patients. Assess LVEF before initiation and then at 3 month intervals of treatment. Withhold TAGRISSO if ejection fraction decreases by 10% from pretreatment values and is less than 50%. For symptomatic congestive heart failure or persistent asymptomatic LV dysfunction that does not resolve within 4 weeks, permanently discontinue TAGRISSO.
- Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during TAGRISSO treatment and for 6 weeks after the final dose. Advise males with female partners of reproductive potential to use effective contraception for 4 months after the final dose.
- The most common adverse reactions (>20%) observed in TAGRISSO patients were diarrhea (42%), rash (41%), dry skin (31%) and nail toxicity (25%).
Please see accompanying complete Prescribing Information including Patient Information.
– ENDS –
NOTES TO EDITORS
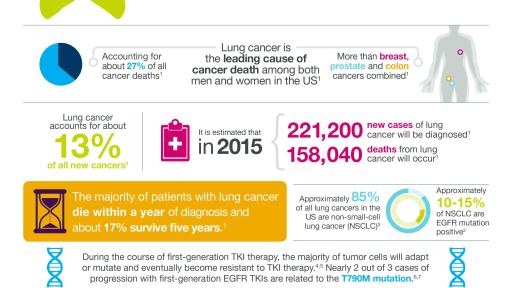
About Non-Small Cell Lung Cancer
Lung cancer is the leading cause of cancer death among both men and women, accounting for about one-third of all cancer deaths, more than breast, prostate and colorectal cancers combined. Lung cancer has a five-year survival rate that is less than 20%. Approximately 85% of all lung cancers in the US are NSCLC; 10% to 15% of these are EGFR mutation-positive. Approximately two-thirds of patients treated with EGFR TKI therapy will acquire resistance related to the T790M mutation.
About TAGRISSO
TAGRISSO™ (osimertinib) (AZD9291) 80mg once-daily tablet is the first medicine indicated for the treatment of metastatic epidermal growth factor receptor (EGFR) T790M mutation-positive NSCLC, as detected by an FDA-approved test, who have progressed on or after EGFR TKI therapy.
About AstraZeneca in Oncology
Oncology is a therapeutic area in which AstraZeneca has deep-rooted heritage. It will be potentially transformational for the company’s future, becoming the sixth growth platform. Our vision is to help patients by redefining the cancer treatment paradigm and one day eliminate cancer as cause of death. By 2020, we are aiming to bring six new cancer medicines to patients.
Our broad pipeline of next-generation medicines is focused on four main disease areas - lung, ovarian, breast, and hematological cancers. These are being targeted through four key platforms – immuno-oncology, the genetic drivers of cancer and resistance, DNA damage repair and antibody drug conjugates.
About Roche
Headquartered in Basel, Switzerland, Roche is a leader in research-focused healthcare with combined strengths in pharmaceuticals and diagnostics. Roche is the world’s largest biotech company, with truly differentiated medicines in oncology, immunology, infectious diseases, ophthalmology and neuroscience. Roche is also the world leader in in vitro diagnostics and tissue-based cancer diagnostics, and a frontrunner in diabetes management. Roche’s personalised healthcare strategy aims at providing medicines and diagnostics that enable tangible improvements in the health, quality of life and survival of patients. Founded in 1896, Roche has been making important contributions to global health for more than a century. Twenty-nine medicines developed by Roche are included in the World Health Organization Model Lists of Essential Medicines, among them life-saving antibiotics, antimalarials and chemotherapy.
About AstraZeneca
AstraZeneca is a global, innovation-driven biopharmaceutical business that focuses on the discovery, development and commercialisation of prescription medicines, primarily for the treatment of cardiovascular, metabolic, respiratory, inflammation, autoimmune, oncology, infection and neuroscience diseases. AstraZeneca operates in over 100 countries and its innovative medicines are used by millions of patients worldwide. For more information please visit www.astrazeneca.com.
CONTACTS
Media Inquiries
Michele Meixell +1 302 885 2677