Media Panel
B-roll
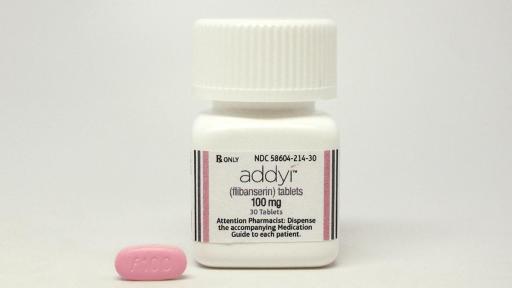
Addyi Packaging
Sprout Pharmaceuticals is located in Raleigh, North Carolina
Cindy Whitehead co-founded and serves as Chief Executive Officer of Sprout
Related Documents
Addyi Prescribing InformationRelated Links
www.sproutpharma.com www.addyi.com Download B-rollSPROUT PHARMACEUTICALS RECEIVES FDA APPROVAL OF ADDYI™ (FLIBANSERIN 100 MG)
First-ever FDA-approved treatment for women’s most common form of sexual dysfunction
PR Newswire, Raleigh, N.C. – August 18, 2015
Sprout Pharmaceuticals, Inc. (Sprout) announced today that the U.S. Food and Drug Administration (FDA) has granted approval of Addyi™ (flibanserin 100 mg) (pronounced add-ee), a once-daily, non-hormonal pill for the treatment of acquired, generalized hypoactive sexual desire disorder (HSDD) in premenopausal women. Addyi is the first and only FDA-approved treatment for this condition, the most common form of female sexual dysfunction, affecting up to 1 in 10 women in the United States.1
“It has been a remarkable journey to get to this breakthrough moment. Today we celebrate what this approval means for all women who have long awaited a medical treatment option for this life impacting condition,” said Cindy Whitehead, chief executive officer of Sprout. “We applaud the FDA for putting the patient voice at the center of the conversation and for focusing on scientific evidence.”
HSDD is defined as a persistent absence of sexual thoughts, fantasies, responsiveness and willingness to engage in sexual activity that causes personal or relationship distress and cannot be accounted for by another medical condition or substance.2 Acquired HSDD refers to HSDD that develops in a patient who previously had no problems with sexual desire. Generalized HSDD refers to HSDD that occurs regardless of the type of stimulation, situation or partner.3
Flibanserin has been studied in more than 11,000 women.4 The FDA approval of Addyi is largely based on three North American, Phase 3, 24-week, randomized double blind, placebo-controlled studies of premenopausal women with HSDD.5 For premenopausal women with HSDD, Addyi has demonstrated improvements in desire for sex, reducing distress from the loss of sexual desire and increasing the number of satisfying sexual events.5
The safety of flibanserin is based on clinical trial data in more than 8,500 women, over 1,000 of which were exposed to treatment for at least one year.4 Addyi was administered to over 2,500 premenopausal women with acquired, generalized HSDD in clinical trials, over 850 receiving treatment for at least 12 months.4 Discontinuation rates due to adverse events in the Phase 3 trials were 13% for Addyi and 6% for placebo. The most common adverse events among patients treated with Addyi were dizziness, somnolence, nausea, fatigue, insomnia and dry mouth. Hypotension and syncope were seen rarely with Addyi alone but more frequently when Addyi was taken in the morning and when co-administered with alcohol or certain other drugs. Alcohol consumption is contraindicated for women taking Addyi.5 With the FDA, Sprout Pharmaceuticals developed a comprehensive Risk Evaluation and Mitigation Strategy (REMS) program, including prescriber and pharmacist certification, to ensure safe use of Addyi. Sprout has also committed to enhanced pharmacovigilance and Phase 4 studies to mitigate and further characterize the safety profile of Addyi.
Addyi is anticipated to be available by October 17, 2015. For more information and Prescribing Information, visit www.addyi.com.
Please see Indication and Important Safety Information below.
Conference Call
Sprout Pharmaceuticals will host a teleconference on Wednesday, August 19, at 9:30 a.m. EDT. The teleconference is accessible by dialing 888-632-3383, with international callers dialing 785-424-1676.
About Sprout Pharmaceuticals
Sprout Pharmaceuticals is passionate about women’s sexual health. With a breakthrough concept for women, the company “sprouted” out of Slate Pharmaceuticals in 2011. Based in Raleigh, N.C., the company is focused solely on the delivery of a treatment option for women with Hypoactive Sexual Desire Disorder (HSDD). For more information or the latest news about Sprout Pharmaceuticals, visit www.sproutpharma.com or call 1-844-PINK-PILL (1-844-746-5745).
Indication and Important Safety Information
Indication
- Addyi is indicated for the treatment of premenopausal women with acquired, generalized hypoactive sexual desire disorder (HSDD) as characterized by low sexual desire that causes marked distress or interpersonal difficulty and is NOT due to:
- – A co-existing medical or psychiatric condition,
- – Problems within the relationship, or
- – The effects of a medication or other drug substance.
Acquired HSDD refers to HSDD that develops in a patient who previously had no problems with sexual desire. Generalized HSDD refers to HSDD that occurs regardless of the type of stimulation, situation or partner.
Limitations of Use
- Addyi is not indicated for the treatment of HSDD in postmenopausal women or in men.
- Addyi is not indicated to enhance sexual performance.
Important Safety Information
WARNING: HYPOTENSION AND SYNCOPE IN CERTAIN SETTINGS
See full prescribing information for complete boxed warning.
- Use of Addyi and alcohol increases the risk of severe hypotension and syncope; therefore alcohol use is contraindicated. Before prescribing Addyi, assess the likelihood of the patient abstaining from alcohol. Counsel patients prescribed Addyi about the importance of abstaining from alcohol.
- Addyi is available only through a restricted program called the Addyi REMS Program.
- Severe hypotension and syncope can occur when Addyi is used with moderate or strong CYP3A4 inhibitors or in patients with hepatic impairment; therefore, Addyi use in these settings is contraindicated.
Contraindications
Addyi is contraindicated:
- With use of alcohol.
- With concomitant use with moderate or strong CYP3A4 inhibitors.
- In patients with hepatic impairment.
Summary of Warnings and Precautions
- Hypotension and Syncope due to an Interaction with Alcohol. An interaction between Addyi and alcohol increases the risk of severe hypotension and syncope. Alcohol use is contraindicated. Before prescribing Addyi, the healthcare provider should assess the likelihood of the patient abstaining from alcohol use.
- Addyi Risk Evaluation and Mitigation Strategy (REMS) Program. Addyi is available only through a restricted program called the Addyi REMS Program, because of the increased risk of severe hypotension and syncope due to an interaction between Addyi and alcohol. The Addyi REMS requires that prescribers are certified by enrolling and completing training; and, pharmacies are certified and will not dispense Addyi unless it is prescribed by a certified prescriber. More information is available at www.AddyiREMS.com.
- Hypotension and Syncope with CYP3A4 Inhibitors.
- Moderate and strong CYP3A4 inhibitors significantly increase Addyi concentrations, which can lead to hypotension and syncope. Concomitant use of Addyi with a moderate or strong CYP3A4 inhibitor is contraindicated.
- Concomitant use of multiple weak CYP3A4 inhibitors that may include herbal supplements (e.g., ginkgo, resveratrol) or non-prescription drugs (e.g., cimetidine) could also lead to clinically relevant increases in flibanserin concentrations that may increase the risk of hypotension and syncope.
- Central Nervous System Depression. Addyi can cause CNS depression (e.g., somnolence, sedation). In five 24-week, randomized, placebo-controlled, double blind trials of premenopausal women with HSDD the incidence of somnolence, sedation or fatigue was 21% and 8% in patients treated with 100 mg of Addyi at bedtime and placebo, respectively. The risk of CNS depression is increased if Addyi is taken during waking hours, or if Addyi is taken with alcohol or other CNS depressants, or with medications that increase flibanserin concentrations. Patients should not drive or engage in other activities requiring full alertness until at least 6 hours after taking Addyi and until they know how Addyi affects them.
- Hypotension and Syncope with Addyi Alone. The use of Addyi – without other concomitant medications known to cause hypotension or syncope - can cause hypotension and syncope. In five 24-week, randomized, placebo-controlled, double-blind trials of premenopausal women with HSDD, hypotension was reported in 0.2% and <0.1% of Addyi-treated patients and placebo-treated patients, respectively; syncope was reported in 0.4% and 0.2% of Addyi-treated patients and placebo-treated patients, respectively. The risk of hypotension and syncope is increased if Addyi is taken during waking hours. Consider the benefits of Addyi and the risks of hypotension and syncope in patients with pre-existing conditions that predispose to hypotension. Patients who experience pre-syncope should immediately lie supine and promptly seek medical help if the symptoms do not resolve. Prompt medical attention should also be obtained for patients who experience syncope.
- Syncope and Hypotension in Patients with Hepatic Impairment. Any degree of hepatic impairment significantly increases flibanserin concentrations, which can lead to hypotension, syncope, and CNS depression. Therefore, Addyi is contraindicated in patients with hepatic impairment.
Most Common Adverse Reactions
- The most common adverse reactions among patients treated with Addyi were dizziness (Addyi 11.4%; Placebo 2.2%), somnolence (Addyi 11.2%; Placebo 2.9%), nausea (Addyi 10.4%; Placebo 3.9%), fatigue (Addyi 9.2%; Placebo 5.5%), insomnia (Addyi 4.9%; Placebo 2.8%) and dry mouth (Addyi 2.4%; Placebo 1.0%).
Summary of Drug Interactions
- Addyi is primarily metabolized by CYP3A4 and, to a lesser extent, by CYP2C19.
- Addyi is contraindicated in women taking a moderate (e.g., fluconazole) or strong (e.g., ketoconazole) CYP3A4 inhibitor.
- Patients using Addyi with combined oral contraceptives or with weak CYP3A4 inhibitors may experience a higher incidence of adverse reactions.
- CYP2C19 inhibitors (e.g. proton pump inhibitors, selective serotonin reuptake inhibitors, benzodiazepines, antifungals) may increase Addyi exposure, which may increase the risk of hypotension, syncope, and CNS depression.
- Do not use Addyi with strong CYP3A4 inducers (e.g., rifampin, St. John’s Wort) as this will substantially reduce the concentration of Addyi.
- Addyi has the potential to inhibit P-glycoprotein (P-gp). Drug concentrations of any narrow therapeutic index drugs that are substrates for P-gp (e.g., digoxin) should be monitored if co-administered with Addyi. The concomitant use of Addyi with digoxin, a drug that is transported by P-gp, increases the digoxin concentration. This may lead to digoxin toxicity.
###
For More Information:
Amy Rose
Edelman
312-297-6912
[email protected]
REFERENCES
1 Lodise, NM. Hypoactive sexual desire disorder in women: treatment options beyond testosterone and approaches to communicating with patients on sexual health. Pharmacotherapy. 2013 Apr;33(4):411-21. doi: 10.1002/phar.1209.
2 Clayton AH, Goldfischer ER, Goldstein I, DeRogatis L, Lewis-D'Agostino D, Pyke R: Validation of the Decreased Sexual Desire Screener (DSDS): a brief diagnostic instrument for generalized acquired female Hypoactive Sexual Desire Disorder (HSDD).J. Sex. Med. 6, 730—738 (2009).
3 American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Psychiatry 2008 55 Fourth Edition, Text Revision. Washington, DC: American Psychiatric Press Inc (2000).
4 Flibanserin For the Treatment of Hypoactive Sexual Desire Disorder in Premenopausal Women Advisory Committee Briefing Document (2015): Accessible via http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DrugSafetyandRiskManagementAdvisoryCommittee/UCM449090.pdf
5 Addyi Prescribing Information
PR-0009.01