For press inquiries:
Audra Friis, Pascale Communications
for Alimera Sciences
917.519.9577
[email protected]
For investor inquiries:
David Burke, The Ruth Group
for Alimera Sciences
646-536-7009
[email protected]
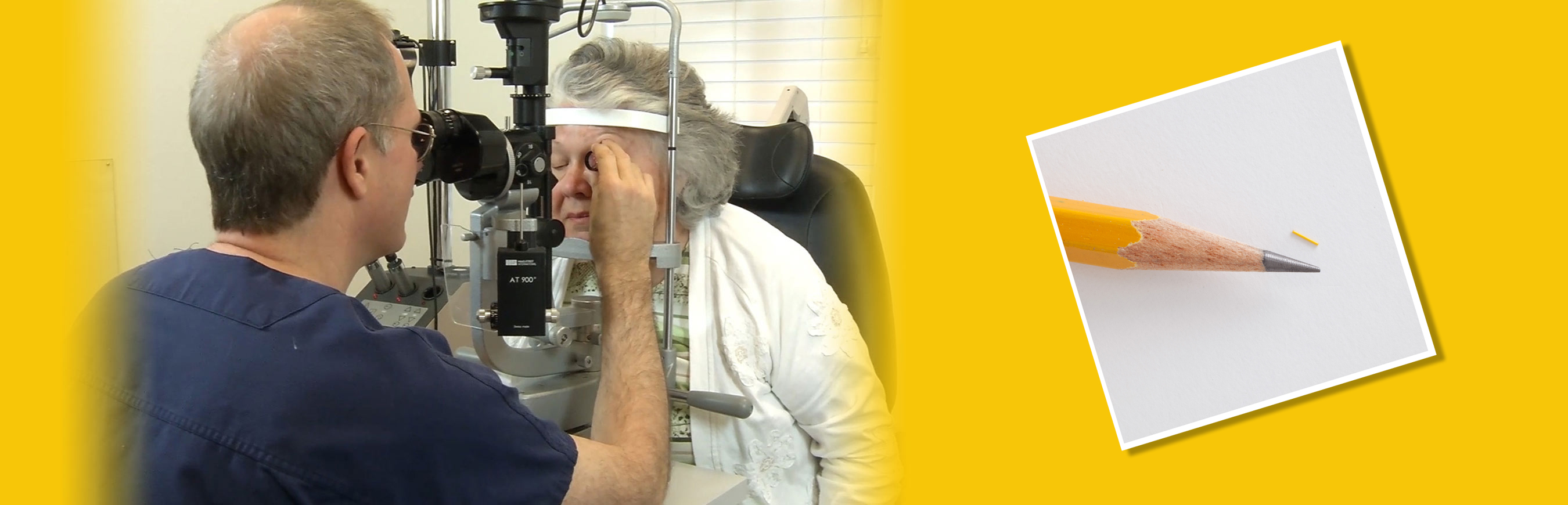
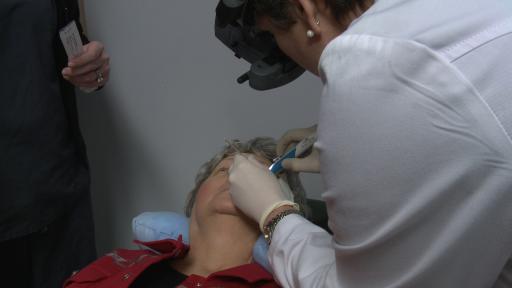
On-demand Launch Event Features the first U.S. Patient Injections of ILUVIEN
Multimedia Gallery
The live web event was supported by eight leading retina specialists and one glaucoma specialist who shared early clinical experiences with ILUVIEN, showed videos of first patient injections, discussed patient case studies, presented clinical information and fielded questions from their colleagues around the country. The webcast is now available for on-demand viewing on Alimera’s product website, www.ILUVIEN.com/webcasts.
“Kicking off the ILUVIEN U.S. commercial launch through a live webcast was an excellent way to quickly and effectively communicate the clinical attributes of ILUVIEN. The excitement and enthusiasm we felt from the physicians, both in the room and online, was quite evident,” said Dan Myers, president and chief executive officer of Alimera. “For those physicians unable to attend the live event, we are pleased to offer them the same opportunity to learn from their peers through the on-demand webinar link on the ILUVIEN website.”
“The level of engagement we felt from our colleagues, both during and after the webcast, is a testament to their eagerness to provide their patients with a multiyear implant that is effective as well as convenient,” said Nancy Holekamp, M.D., retina specialist at Pepose Vision, Chesterfield, Missouri, and moderator of the webcast event. “It is not surprising that the product has been so well embraced by both the physician and patient communities. The feedback from patients involved in the initial round of injections has been extremely encouraging.”
In addition to Dr. Holekamp, other webinar presenters included: Dr. David Brown, Retina Consultants of Houston, Houston, Texas; Dr. Pravin Dugel, Retinal Consultants of Arizona, Phoenix, Arizona; Dr. Alexander Eaton, Retina Health Center, Fort Myers, Florida; Dr. Victor Gonzalez, Valley Retina Institute, McAllen, Texas; Dr. Peter Kaiser, Cole Eye Institute, Cleveland Clinic, Cleveland, Ohio; Dr. Szilàrd Kiss, Weill Cornell Medical College, New York-Presbyterian Hospital, New York, New York; Dr. Baruch Kuppermann, U.C. Irvine Medical Center, Orange, California; and Dr. Thomas Mundorf, Mundorf Eye Center, Novant Health Presbyterian Medical Center, Charlotte, North Carolina.
“Many of our patients are tethered to their doctors’ offices by the need for frequent injections to maintain their visual acuity. As an implant that provides drug delivery over multiple years with just one injection, ILUVIEN could make a big improvement in their treatment regimen,” said Dr. Dugel. “I believe interest in ILUVIEN as an effective treatment option for DME will continue to grow as patients experience visual improvement as a result of the implant.”
About ILUVIEN
www.ILUVIEN.com
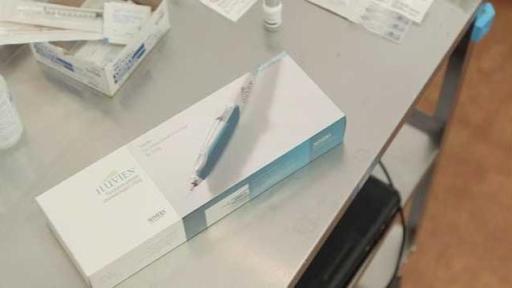
ILUVIEN® (fluocinolone acetonide intravitreal implant) 0.19 mg is a sustained release intravitreal implant approved in the U.S. to treat diabetic macular edema (DME) in patients who have been previously treated with a course of corticosteroids and did not have a clinically significant rise in intraocular pressure. Each ILUVIEN implant is designed to release submicrogram levels of fluocinolone acetonide (FAc), a corticosteroid, for 36 months.
Corticosteroids have a history of effective use in treating ocular disease inflammation. ILUVIEN is injected in the back of the patient’s eye with an applicator that employs a 25-gauge needle, which allows for a self-sealing wound. In the FAME™ Study, a phase 3 clinical study of ILUVIEN, the primary endpoint of improvement in vision of 15 letters or more was demonstrated at 24 months and the most frequently reported adverse drug reactions included cataract development and increased ocular pressure.
ILUVIEN Important Safety Information
Contraindications
- ILUVIEN is contraindicated in patients with active or suspected ocular or periocular infections including most viral disease of the cornea and conjunctiva, including active epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, varicella, mycobacterial infections and fungal diseases.
- ILUVIEN is contraindicated in patients with glaucoma, who have cup to disc ratios of greater than 0.8.
- ILUVIEN is contraindicated in patients with known hypersensitivity to any components of this product.
Warnings and Precautions
- Intravitreal injections have been associated with endophthalmitis, eye inflammation, increased intraocular pressure, and retinal detachments. Patients should be monitored following the injection.
- Use of corticosteroids may produce posterior subcapsular cataracts, increased intraocular pressure, glaucoma, and may enhance the establishment of secondary ocular infections due to bacteria, fungi, or viruses. Corticosteroids are not recommended to be used in patients with a history of ocular herpes simplex because of the potential for reactivation of the viral infection.
- Patients in whom the posterior capsule of the lens is absent or has a tear are at risk of implant migration into the anterior chamber.
Adverse Reactions
- In controlled studies, the most common adverse reactions reported were cataract development (ILUVIEN 82%; sham 50%) and intraocular pressure elevation of greater than10 mmHg (ILUVIEN 34%; sham 10%).
Patients are advised to have follow-up eye examinations at appropriate intervals following treatment with ILUVIEN. For full prescribing information, log onto www.ILUVIEN.com.
To report suspected adverse reactions, contact Alimera Sciences, Inc. at 1-844-445-8843 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
About Diabetic Macular Edema (DME)
DME, the primary cause of vision loss associated with diabetic retinopathy, is a disease affecting the macula, the part of the retina responsible for central vision. When the blood vessel leakage associated with diabetic retinopathy results in swelling of the macula, the condition is called DME. The onset of DME is painless and may go unreported by the patient until it manifests with the blurring of central vision or acute vision loss. The severity of this blurring may range from mild to profound loss of vision. The Wisconsin Epidemiologic Study of Diabetic Retinopathy found that over a 10-year period approximately 19% of people with diabetes included in the study were diagnosed with DME. All people with type 1 or type 2 diabetes are at risk of developing DME. As the population of people with diabetes increases, Alimera expects the annual incidence of diagnosed DME to increase, as well.
About Alimera Sciences, Inc.
www.alimerasciences.com Alimera Sciences, Inc., headquartered in Alpharetta, Georgia, is a pharmaceutical company that specializes in the research, development and commercialization of prescription ophthalmic pharmaceuticals. Alimera’s European operations are conducted from London by its subsidiary, Alimera Sciences Limited.
Forward Looking Statements
This press release contains “forward-looking statements,” within the meaning of the Private Securities Litigation Reform Act of 1995, regarding, among other things, the availability of ILUVIEN in the U.S. and assumptions regarding the prevalence of DME in the U.S. Such forward-looking statements are based on current expectations and involve inherent risks and uncertainties, including factors that could delay, divert or change any of them, and could cause actual results to differ materially from those projected in its forward-looking statements. Meaningful factors which could cause actual results to differ include, but are not limited to, uncertainty as to Alimera’s ability to commercialize, and market acceptance of, ILUVIEN in the U.S., as well as other factors discussed in the “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of Alimera’s Annual Report on Form 10-K for the year ended December 31, 2014, which is on file with the Securities and Exchange Commission (SEC) and available on the SEC’s website at www.sec.gov. Additional factors may also be set forth in those sections of Alimera’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2015 to be filed with the SEC. In addition to the risks described above and in Alimera’s Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and other filings with the SEC, other unknown or unpredictable factors also could affect Alimera’s results. There can be no assurance that the actual results or developments anticipated by Alimera will be realized or, even if substantially realized, that they will have the expected consequences to, or effects on, Alimera. Therefore, no assurance can be given that the outcomes stated in such forward-looking statements and estimates will be achieved.
All forward-looking statements contained in this press release are expressly qualified by the cautionary statements contained or referred to herein. Alimera cautions investors not to rely too heavily on the forward-looking statements Alimera makes or that are made on its behalf. These forward-looking statements speak only as of the date of this press release (unless another date is indicated). Alimera undertakes no obligation, and specifically declines any obligation, to publicly update or revise any such forward-looking statements, whether as a result of new information, future events or otherwise.
###