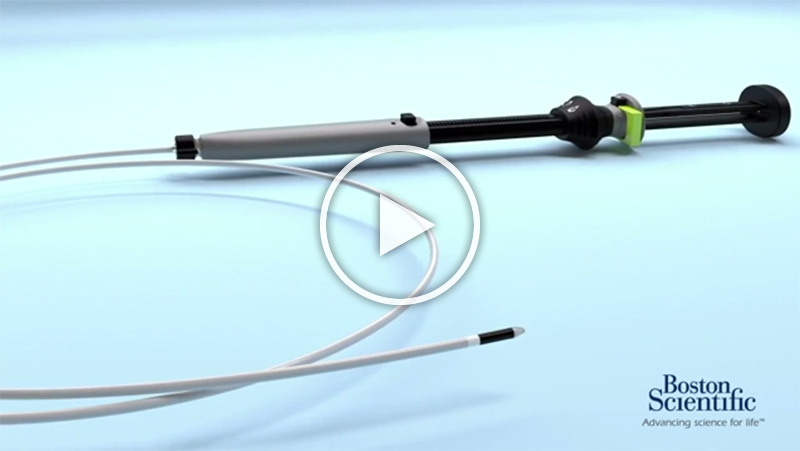
Boston Scientific Launches the AXIOS™ Stent and Electrocautery Enhanced Delivery System
PR Newswire, MARLBOROUGH, Mass. (March 22, 2016)
Boston Scientific Corporation (NYSE: BSX) today announced the launch of the AXIOS™ Stent and Electrocautery Enhanced Delivery System to help physicians manage two serious complications from pancreatitis using a minimally invasive endoscopic approach.
The AXIOS System has been cleared for endoscopic management of pancreatic pseudocysts and certain types of walled-off pancreatic necrosis.1 These conditions represent two types of pancreatic fluid collections (PFCs) that occur in 5-16 percent of patients with acute pancreatitis and 20-40 percent of patients with chronic pancreatitis.2 While some of these PFCs are asymptomatic and self-resolving, others cause severe symptoms and require treatment.
Under endoscopic ultrasound (EUS) guidance, a physician can utilize the AXIOS System electrocautery-enhanced catheter to gain access to the PFC and deploy the AXIOS stent which facilitates drainage of the PFC by creating a temporary channel between the PFC and the gastrointestinal tract. The large flanges on each end of the lumen-apposing stent reduce the risk of leakage and migration. The stent – which is MRI compatible, fully covered, and self-expanding – is the only removable metal stent in the U.S. indicated for PFC drainage.
Although symptomatic PFCs can also be treated surgically, surgery has been associated with high rates of morbidity (7–37 percent) and mortality (6 percent).2 Endoscopic solutions may offer a less invasive treatment option associated with shorter hospital stays, better physical and mental health of patients, as well as lower cost.3
“The AXIOS Stent and Electrocautery Enhanced Delivery System provide a simpler and faster treatment option for patients,” said Kenneth Binmoeller, M.D., California Pacific Medical Center, San Francisco, CA and the inventor of the AXIOS System. “We can now provide an endoscopic solution that provides immediate relief for these patients using one device in a single setting.”
“In our practice, the AXIOS System has improved the endoscopic treatment of PFCs and walled off necrosis,” said Todd Baron, M.D., professor of Medicine and director of Advanced Therapeutic Endoscopy, University of North Carolina Hospital, Chapel Hill, North Carolina. “The delivery system has improved our procedural efficiency by reducing procedure time and patient exposure to X-ray imaging. In addition, we believe that the large diameter stent design is helping to reduce the cost of care by decreasing hospital length of stay and the number of interventions needed to manage this complex disease.”
For more information, please visit the AXIOS™ System product page here. Or follow Boston Scientific Endoscopy on Twitter at @bsc_endoscopy.
About Boston Scientific
Boston Scientific transforms lives through innovative medical solutions that improve the health of patients around the world. As a global medical technology leader for more than 35 years, we advance science for life by providing a broad range of high performance solutions that address unmet patient needs and reduce the cost of healthcare. For more information, visit www.bostonscientific.com and connect on Twitter and Facebook.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements may be identified by words like "anticipate," "expect," "project," "believe," "plan," "estimate," "intend" and similar words. These forward-looking statements are based on our beliefs, assumptions and estimates using information available to us at the time and are not intended to be guarantees of future events or performance. These forward-looking statements include, among other things, statements regarding new product launches, markets for our products, and product performance and impact. If our underlying assumptions turn out to be incorrect, or if certain risks or uncertainties materialize, actual results could vary materially from the expectations and projections expressed or implied by our forward-looking statements. These factors, in some cases, have affected and in the future (together with other factors) could affect our ability to implement our business strategy and may cause actual results to differ materially from those contemplated by the statements expressed in this press release. As a result, readers are cautioned not to place undue reliance on any of our forward-looking statements.
Factors that may cause such differences include, among other things: future economic, competitive, reimbursement and regulatory conditions; new product introductions; demographic trends; intellectual property; litigation; financial market conditions; and future business decisions made by us and our competitors. All of these factors are difficult or impossible to predict accurately and many of them are beyond our control. For a further list and description of these and other important risks and uncertainties that may affect our future operations, see Part I, Item 1A – Risk Factors in our most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission, which we may update in Part II, Item 1A – Risk Factors in Quarterly Reports on Form 10-Q we have filed or will file hereafter. We disclaim any intention or obligation to publicly update or revise any forward-looking statements to reflect any change in our expectations or in events, conditions or circumstances on which those expectations may be based, or that may affect the likelihood that actual results will differ from those contained in the forward-looking statements. This cautionary statement is applicable to all forward-looking statements contained in this document.
CONTACTS:
Tom Keppeler
508-683-6585 (office)
Media Relations
Boston Scientific Corporation
[email protected]
Investors: Susie Lisa, CFA
508-683-5565 (office)
Investor Relations
Boston Scientific Corporation
[email protected]
1 The walled-off necrosis indication for the AXIOS Stent and Electrocautery Enhanced Delivery System is for PFCs containing less than 30 percent dead tissue.
2 Safety and Efficacy of Endoscopic Ultrasound-Guided Drainage of Pancreatic Fluid Collections With Lumen-Apposing Covered Self-Expanding Metal Stents. Shah RJ, Shah JN, Waxman I, Kowalski TE, Sanchez-Yague A, Nieto J, Brauer BC, Gaidhane M, Kahaleh M. Clin Gastroenterol and Hepatol 2014 Oct 5 http://www.ncbi.nlm.nih.gov/pubmed/25290534
3 Varadarajulu et al, Equal Efficacy of Endoscopic and Surgical Cystogastrostomy for Pancreatic Pseudocyst Drainage in a Randomized Trial. Gastroenterology 2013; 145: